High-risk medulloblastoma: a pediatric oncology group randomized trial of chemotherapy before or after radiation therapy (POG 9031)
- PMID: 23857975
- PMCID: PMC3732312
- DOI: 10.1200/JCO.2012.43.9984
High-risk medulloblastoma: a pediatric oncology group randomized trial of chemotherapy before or after radiation therapy (POG 9031)
Abstract
Purpose: To compare event-free survival (EFS) in children with high-risk medulloblastoma randomly assigned to receive either chemotherapy before radiation or chemotherapy after radiation.
Patients and methods: One hundred twelve patients were randomly assigned to each arm. Criteria used to categorize patients as high risk included M1-4 disease by modified Chang staging classification, T3b/T4 disease, or greater than 1.5 cm3 of residual tumor after surgery. Postoperatively, children with high-risk medulloblastoma were randomly assigned to two arms, either chemotherapy entailing three cycles of cisplatin and etoposide before radiation (chemotherapy first [CT1]) or the same chemotherapy regimen after radiation (radiation therapy first [RT1]). Both groups received consolidation chemotherapy consisting of vincristine and cyclophosphamide.
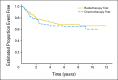
Results: The median follow-up time was 6.4 years. Five-year EFS was 66.0% in the CT1 arm and 70.0% in the RT1 arm (P = .54), and 5-year overall survival in the two groups was 73.1% and 76.1%, respectively (P = .47). In the CT1 arm, 40 of the 62 patients with residual disease achieved either complete or partial remission.
Conclusion: Five-year EFS did not differ significantly whether, after surgery, patients received chemotherapy before or after radiotherapy.
Trial registration: ClinicalTrials.gov NCT00003573.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


References
-
- Gurney JG, Davis S, Severson RK, et al. Trends in cancer incidence among children in the U.S. Cancer. 1996;78:532–541. - PubMed
-
- McNeil DE, Coté TR, Clegg L, et al. Incidence and trends in pediatric malignancies medulloblastoma/primitive neuroectodermal tumor: A SEER update—Surveillance Epidemiology and End Results. Med Pediatr Oncol. 2002;39:190–194. - PubMed
-
- Polkinghorn WR, Tarbell NJ. Medulloblastoma: Tumorigenesis, current clinical paradigm, and efforts to improve risk stratification. Nat Clin Pract Oncol. 2007;4:295–304. - PubMed
-
- Evans AE, Jenkin RD, Sposto R, et al. The treatment of medulloblastoma: Results of a prospective randomized trial of radiation therapy with and without CCNU, vincristine, and prednisone. J Neurosurg. 1990;72:572–582. - PubMed
-
- Tait DM, Thornton-Jones H, Bloom HJ, et al. Adjuvant chemotherapy for medulloblastoma: The first multi-centre control trial of the International Society of Paediatric Oncology (SIOP I) Eur J Cancer. 1990;26:464–469. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

