Pomalidomide is nonteratogenic in chicken and zebrafish embryos and nonneurotoxic in vitro
- PMID: 23858438
- PMCID: PMC3732931
- DOI: 10.1073/pnas.1307684110
Pomalidomide is nonteratogenic in chicken and zebrafish embryos and nonneurotoxic in vitro
Abstract
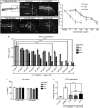
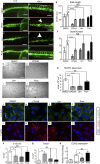
Thalidomide and its analog, Lenalidomide, are in current use clinically for treatment of multiple myeloma, complications of leprosy and cancers. An additional analog, Pomalidomide, has recently been licensed for treatment of multiple myeloma, and is purported to be clinically more potent than either Thalidomide or Lenalidomide. Using a combination of zebrafish and chicken embryos together with in vitro assays we have determined the relative anti-inflammatory activity of each compound. We demonstrate that in vivo embryonic assays Pomalidomide is a significantly more potent anti-inflammatory agent than either Thalidomide or Lenalidomide. We tested the effect of Pomalidomide and Lenalidomide on angiogenesis, teratogenesis, and neurite outgrowth, known detrimental effects of Thalidomide. We found that Pomalidomide, displays a high degree of cell specificity, and has no detectable teratogenic, antiangiogenic or neurotoxic effects at potent anti-inflammatory concentrations. This is in marked contrast to Thalidomide and Lenalidomide, which had detrimental effects on blood vessels, nerves, and embryonic development at anti-inflammatory concentrations. This work has implications for Pomalidomide as a treatment for conditions Thalidomide and Lenalidomide treat currently.
Keywords: CPS49; Cox2; cytoskeleton; drug screening.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Pomalidomide is teratogenic in rats and rabbits and can be neurotoxic in humans.Proc Natl Acad Sci U S A. 2013 Dec 10;110(50):E4819. doi: 10.1073/pnas.1317084110. Epub 2013 Dec 3. Proc Natl Acad Sci U S A. 2013. PMID: 24302769 Free PMC article. No abstract available.
-
Pomalidomide is strongly antiangiogenic and teratogenic in relevant animal models.Proc Natl Acad Sci U S A. 2013 Dec 10;110(50):E4818. doi: 10.1073/pnas.1315875110. Epub 2013 Dec 3. Proc Natl Acad Sci U S A. 2013. PMID: 24302770 Free PMC article. No abstract available.
-
Reply to D’Amato et al. and Zeldis et al.: Screening of thalidomide derivatives in chicken and zebrafish embryos.Proc Natl Acad Sci U S A. 2013 Dec 10;110(50):E4820. doi: 10.1073/pnas.1318475110. Proc Natl Acad Sci U S A. 2013. PMID: 24471177 Free PMC article. No abstract available.
Similar articles
-
Pomalidomide is teratogenic in rats and rabbits and can be neurotoxic in humans.Proc Natl Acad Sci U S A. 2013 Dec 10;110(50):E4819. doi: 10.1073/pnas.1317084110. Epub 2013 Dec 3. Proc Natl Acad Sci U S A. 2013. PMID: 24302769 Free PMC article. No abstract available.
-
Pomalidomide is strongly antiangiogenic and teratogenic in relevant animal models.Proc Natl Acad Sci U S A. 2013 Dec 10;110(50):E4818. doi: 10.1073/pnas.1315875110. Epub 2013 Dec 3. Proc Natl Acad Sci U S A. 2013. PMID: 24302770 Free PMC article. No abstract available.
-
Reply to D’Amato et al. and Zeldis et al.: Screening of thalidomide derivatives in chicken and zebrafish embryos.Proc Natl Acad Sci U S A. 2013 Dec 10;110(50):E4820. doi: 10.1073/pnas.1318475110. Proc Natl Acad Sci U S A. 2013. PMID: 24471177 Free PMC article. No abstract available.
-
Implementation of a Pregnancy Prevention Programme (PPP) with a Controlled Distribution System (CDS) for the Generic Teratogenic Phthalimides Thalidomide, Lenalidomide and Pomalidomide.Ther Innov Regul Sci. 2021 Nov;55(6):1155-1164. doi: 10.1007/s43441-021-00327-3. Epub 2021 Jul 30. Ther Innov Regul Sci. 2021. PMID: 34331266 Review.
-
Pomalidomide for patients with multiple myeloma.Drugs Today (Barc). 2013 Sep;49(9):555-62. doi: 10.1358/dot.2013.49.9.2017031. Drugs Today (Barc). 2013. PMID: 24086951 Review.
Cited by
-
Novel insights into the mechanism of action of lenalidomide.Oncoimmunology. 2014 Feb 1;3:e28386. doi: 10.4161/onci.28386. eCollection 2014. Oncoimmunology. 2014. PMID: 25340011 Free PMC article. No abstract available.
-
A New Generation of IMiDs as Treatments for Neuroinflammatory and Neurodegenerative Disorders.Biomolecules. 2023 Apr 26;13(5):747. doi: 10.3390/biom13050747. Biomolecules. 2023. PMID: 37238617 Free PMC article. Review.
-
Role of chronic neuroinflammation in neuroplasticity and cognitive function: A hypothesis.Alzheimers Dement. 2022 Nov;18(11):2327-2340. doi: 10.1002/alz.12610. Epub 2022 Mar 2. Alzheimers Dement. 2022. PMID: 35234334 Free PMC article.
-
Thalidomide-induced teratogenesis: history and mechanisms.Birth Defects Res C Embryo Today. 2015 Jun;105(2):140-56. doi: 10.1002/bdrc.21096. Epub 2015 Jun 4. Birth Defects Res C Embryo Today. 2015. PMID: 26043938 Free PMC article. Review.
-
Pomalidomide Improves Motor Behavioral Deficits and Protects Cerebral Cortex and Striatum Against Neurodegeneration Through a Reduction of Oxidative/Nitrosative Damages and Neuroinflammation After Traumatic Brain Injury.Cell Transplant. 2024 Jan-Dec;33:9636897241237049. doi: 10.1177/09636897241237049. Cell Transplant. 2024. PMID: 38483119 Free PMC article.
References
-
- Vargesson N. Thalidomide-induced limb defects: Resolving a 50-year-old puzzle. Bioessays. 2009;31(12):1327–1336. - PubMed
-
- Franks ME, Macpherson GR, Figg WD. Thalidomide. Lancet. 2004;363(9423):1802–1811. - PubMed
-
- Delforge M, et al. Treatment-related peripheral neuropathy in multiple myeloma: the challenge continues. Lancet Oncol. 2010;11(11):1086–1095. - PubMed
-
- Richardson PG, et al. Management of treatment-emergent peripheral neuropathy in multiple myeloma. Leukemia. 2012;26(4):595–608. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

