The deoxynucleotide triphosphohydrolase SAMHD1 is a major regulator of DNA precursor pools in mammalian cells
- PMID: 23858451
- PMCID: PMC3761606
- DOI: 10.1073/pnas.1312033110
The deoxynucleotide triphosphohydrolase SAMHD1 is a major regulator of DNA precursor pools in mammalian cells
Abstract
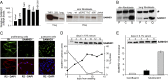
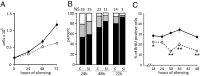
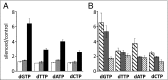
Sterile alpha motif and HD-domain containing protein 1 (SAMHD1) is a triphosphohydrolase converting deoxynucleoside triphosphates (dNTPs) to deoxynucleosides. The enzyme was recently identified as a component of the human innate immune system that restricts HIV-1 infection by removing dNTPs required for viral DNA synthesis. SAMHD1 has deep evolutionary roots and is ubiquitous in human organs. Here we identify a general function of SAMHD1 in the regulation of dNTP pools in cultured human cells. The protein was nuclear and variably expressed during the cell cycle, maximally during quiescence and minimally during S-phase. Treatment of lung or skin fibroblasts with specific siRNAs resulted in the disappearence of SAMHD1 accompanied by loss of the cell-cycle regulation of dNTP pool sizes and dNTP imbalance. Cells accumulated in G1 phase with oversized pools and stopped growing. Following removal of the siRNA, the pools were normalized and cell growth restarted, but only after SAMHD1 had reappeared. In quiescent cultures SAMHD1 down-regulation leads to a marked expansion of dNTP pools. In all cases the largest effect was on dGTP, the preferred substrate of SAMHD1. Ribonucleotide reductase, responsible for the de novo synthesis of dNTPs, is a cytosolic enzyme maximally induced in S-phase cells. Thus, in mammalian cells the cell cycle regulation of the two main enzymes controlling dNTP pool sizes is adjusted to the requirements of DNA replication. Synthesis by the reductase peaks during S-phase, and catabolism by SAMHD1 is maximal during G1 phase when large dNTP pools would prevent cells from preparing for a new round of DNA replication.
Keywords: cell cycle arrest; dGTP pool; dNTP regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Comment in
-
Deoxynucleoside triphosphate (dNTP) synthesis and destruction regulate the replication of both cell and virus genomes.Proc Natl Acad Sci U S A. 2013 Aug 27;110(35):14120-1. doi: 10.1073/pnas.1312901110. Epub 2013 Aug 14. Proc Natl Acad Sci U S A. 2013. PMID: 23946423 Free PMC article. No abstract available.
References
-
- Li N, Zhang W, Cao X. Identification of human homologue of mouse IFN-gamma induced protein from human dendritic cells. Immunol Lett. 2000;74(3):221–224. - PubMed
-
- Qiao F, Bowie JU. The many faces of SAM. Sci STKE. 2005;2005(286):re7. - PubMed
-
- Aravind L, Koonin EV. The HD domain defines a new superfamily of metal-dependent phosphohydrolases. Trends Biochem Sci. 1998;23(12):469–472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

