The effect of ligand lipophilicity on the nanoparticle encapsulation of Pt(IV) prodrugs
- PMID: 23859129
- PMCID: PMC3773226
- DOI: 10.1021/ic4010642
The effect of ligand lipophilicity on the nanoparticle encapsulation of Pt(IV) prodrugs
Abstract
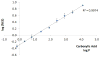
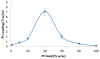
In an effort to expand the therapeutic range of platinum anticancer agents, several new approaches to platinum-based therapy, including nanodelivery, are under active investigation. To better understand the effect of ligand lipophilicity on the encapsulation of Pt(IV) prodrugs within polymer nanoparticles, the series of compounds cis,cis,trans-[Pt(NH3)2Cl2L2] was prepared, where L = acetate, propanoate, butanoate, pentanoate, hexanoate, heptanoate, octanoate, nonanoate, and decanoate. The lipophilicities of these compounds, assessed by reversed-phase HPLC, correlate with the octanol/water partition coefficients of their respective free carboxylic acid ligands, which in turn affect the degree of encapsulation of the Pt(IV) complex within the hydrophobic core of poly(lactic-co-glycolic acid)-block-poly(ethylene glycol) (PLGA-PEG-COOH) nanoparticles. The most lipophilic compound investigated, cis,cis,trans-[Pt(NH3)2Cl2(O2C(CH2)8CH3)2], displayed the best encapsulation. This compound was therefore selected to evaluate the effect of increased platinum concentration on encapsulation. As the platinum concentration was increased, there was an initial increase in encapsulation followed by a decrease due to macroscopic precipitation. Maximal loading occurred when the platinum complex was present at a 40% w/w ratio with respect to polymer during the nanoprecipitation step. Particles formed under these optimal conditions had diameters of approximately 50 nm, as determined by transmission electron microscopy.
Figures





References
-
- Rosenberg B, VanCamp L, Trosko JE, Mansour VH. Nature. 1969;222:385–386. - PubMed
-
- Wheate NJ, Walker S, Craig GE, Oun R. Dalton Trans. 2010;39:8113–8127. - PubMed
-
- O’Dwyer PJ, Stevenson JP, Johnson SW. Clinical Status of Cisplatin, Carboplatin, and Other Platinum-Based Antitumor Drugs. In: Lippert B, editor. Cisplatin - Chemistry and Biochemistry of a Leading Anticancer Drug. Verlag Helvetica Chimica Acta; Zürich, Switzerland: 1999. pp. 31–69.
-
- Kelland L. Nat Rev Cancer. 2007;7:573–584. - PubMed
-
- Farokhzad OC, Langer R. ACS Nano. 2009;3:16–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

