Hemodialysis and water quality
- PMID: 23859187
- PMCID: PMC4596525
- DOI: 10.1111/sdi.12113
Hemodialysis and water quality
Abstract
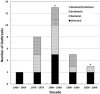
Over 383,900 individuals in the U.S. undergo maintenance hemodialysis that exposes them to water, primarily in the form of dialysate. The quality of water and associated dialysis solutions have been implicated in adverse patient outcomes and is therefore critical. The Association for the Advancement of Medical Instrumentation has published both standards and recommended practices that address both water and the dialyzing solutions. Some of these recommendations have been adopted into Federal Regulations by the Centers for Medicare and Medicaid Services as part of the Conditions for Coverage, which includes limits on specific contaminants within water used for dialysis, dialysate, and substitution fluids. Chemical, bacterial, and endotoxin contaminants are health threats to dialysis patients, as shown by the continued episodic nature of outbreaks since the 1960s causing at least 592 cases and 16 deaths in the U.S. The importance of the dialysis water distribution system, current standards and recommendations, acceptable monitoring methods, a review of chemical, bacterial, and endotoxin outbreaks, and infection control programs are discussed.
Published 2013. This article is a U.S. Government work and is in the public domain in the USA.
Figures
References
-
- USRDS . U.S. Renal Data System, USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2012.
-
- AAMI . TIR43: ultrapure dialysate for hemodialysis and related therapies. Association for the Advancement of Medical Instrumentation; Arlington, VA: 2011.
-
- Ward RA. Avoiding toxicity from water-borne contaminants in hemodialysis: new challenges in an era of increased demand for water. Adv Chronic Kidney Dis. 2011;18:207–213. - PubMed
-
- Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. J Am Med Assoc. 2007;298:2038–2047. - PubMed
-
- HHS CMS . Conditions for Coverage for End-Stage Renal Disease Facilities. 42 CFR Parts 405, 410, 413, 414, 488, and 494. Department of Human and Health Services, Centers for Medicare and Medicaid Services; Baltimore, MD: 2008.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

