Transition-state inhibitors of purine salvage and other prospective enzyme targets in malaria
- PMID: 23859211
- PMCID: PMC3819805
- DOI: 10.4155/fmc.13.51
Transition-state inhibitors of purine salvage and other prospective enzyme targets in malaria
Abstract
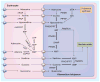
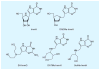
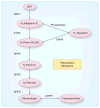
Malaria is a leading cause of human death within the tropics. The gradual generation of drug resistance imposes an urgent need for the development of new and selective antimalarial agents. Kinetic isotope effects coupled to computational chemistry have provided the relevant details on geometry and charge of enzymatic transition states to facilitate the design of transition-state analogs. These features have been reproduced into chemically stable mimics through synthetic chemistry, generating inhibitors with dissociation constants in the pico- to femto-molar range. Transition-state analogs are expected to contribute to the control of malaria.
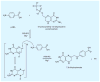
Figures








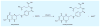


Similar articles
-
Synthesis of purine N9-[2-hydroxy-3-O-(phosphonomethoxy)propyl] derivatives and their side-chain modified analogs as potential antimalarial agents.Bioorg Med Chem. 2012 Feb 1;20(3):1222-30. doi: 10.1016/j.bmc.2011.12.034. Epub 2011 Dec 30. Bioorg Med Chem. 2012. PMID: 22249123
-
Plasmodium falciparum: new molecular targets with potential for antimalarial drug development.Expert Rev Anti Infect Ther. 2009 Nov;7(9):1087-98. doi: 10.1586/eri.09.93. Expert Rev Anti Infect Ther. 2009. PMID: 19883329 Review.
-
Acyclic phosph(on)ate inhibitors of Plasmodium falciparum hypoxanthine-guanine-xanthine phosphoribosyltransferase.Bioorg Med Chem. 2013 Sep 1;21(17):5629-46. doi: 10.1016/j.bmc.2013.02.016. Epub 2013 Mar 5. Bioorg Med Chem. 2013. PMID: 23810424 Free PMC article.
-
Synthesis of branched 9-[2-(2-phosphonoethoxy)ethyl]purines as a new class of acyclic nucleoside phosphonates which inhibit Plasmodium falciparum hypoxanthine-guanine-xanthine phosphoribosyltransferase.Bioorg Med Chem. 2009 Sep 1;17(17):6218-32. doi: 10.1016/j.bmc.2009.07.044. Epub 2009 Jul 25. Bioorg Med Chem. 2009. PMID: 19666228
-
Purine and pyrimidine pathways as targets in Plasmodium falciparum.Curr Top Med Chem. 2011;11(16):2103-15. doi: 10.2174/156802611796575948. Curr Top Med Chem. 2011. PMID: 21619511 Free PMC article. Review.
Cited by
-
Genetic resistance to purine nucleoside phosphorylase inhibition in Plasmodium falciparum.Proc Natl Acad Sci U S A. 2018 Feb 27;115(9):2114-2119. doi: 10.1073/pnas.1525670115. Epub 2018 Feb 12. Proc Natl Acad Sci U S A. 2018. PMID: 29440412 Free PMC article.
-
Pinpointing dynamic coupling in enzymes for efficient drug design.Future Sci OA. 2016 Jan 25;2(1):FSO95. doi: 10.4155/fsoa.2015.0017. eCollection 2016 Mar. Future Sci OA. 2016. PMID: 28031945 Free PMC article. No abstract available.
-
Interaction of Tri-Cyclic Nucleobase Analogs with Enzymes of Purine Metabolism: Xanthine Oxidase and Purine Nucleoside Phosphorylase.Int J Mol Sci. 2024 Sep 27;25(19):10426. doi: 10.3390/ijms251910426. Int J Mol Sci. 2024. PMID: 39408755 Free PMC article.
-
A resistant mutant of Plasmodium falciparum purine nucleoside phosphorylase uses wild-type neighbors to maintain parasite survival.J Biol Chem. 2021 Jan-Jun;296:100342. doi: 10.1016/j.jbc.2021.100342. Epub 2021 Jan 30. J Biol Chem. 2021. PMID: 33524395 Free PMC article.
-
Substrate and Inhibitor Specificity of the Plasmodium berghei Equilibrative Nucleoside Transporter Type 1.Mol Pharmacol. 2016 Jun;89(6):678-85. doi: 10.1124/mol.115.101386. Epub 2016 Apr 5. Mol Pharmacol. 2016. PMID: 27048953 Free PMC article.
References
-
- Svenson JE, MacLean JD, Gyorkos TW, Keystone J. Imported malaria. Clinical presentation and examination of symptomatic travelers. Arch Intern Med. 1995;155(8):861–868. - PubMed
-
- Holt RA, Subramanian GM, Halpern A, et al. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298(5591):129–149. - PubMed
-
- White NJ, Breman JG. Malaria. In: Anthony SF, Dennis LK, Dan LL, et al., editors. Harrison’s Principles of Internal Medicine. The McGraw-Hill Companies; NY, USA: 2008. pp. 1280–1294.
-
- Foley M, Tilley L. Quinoline antimalarials: mechanisms of action and resistance and prospects for new agents. Pharmacol Ther. 1998;79(1):55–87. - PubMed
Websites
-
- Centers for Disease Control and Prevention. Malaria Biology. www.cdc.gov/malaria/about/biology.
-
- WHO. World Malaria Report. 2011 www.who.int/malaria/world_malaria_report_2011/en.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
