Radical-based regioselective C-H functionalization of electron-deficient heteroarenes: scope, tunability, and predictability
- PMID: 23859263
- PMCID: PMC3776592
- DOI: 10.1021/ja406223k
Radical-based regioselective C-H functionalization of electron-deficient heteroarenes: scope, tunability, and predictability
Abstract
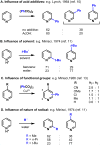
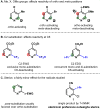
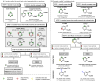
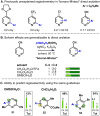
Radical addition processes can be ideally suited for the direct functionalization of heteroaromatic bases, yet these processes are only sparsely used due to the perception of poor or unreliable control of regiochemistry. A systematic investigation of factors affecting the regiochemistry of radical functionalization of heterocycles using alkylsulfinate salts revealed that certain types of substituents exert consistent and additive effects on the regioselectivity of substitution. This allowed us to establish guidelines for predicting regioselectivity on complex π-deficient heteroarenes, including pyridines, pyrimidines, pyridazines, and pyrazines. Since the relative contribution from opposing directing factors was dependent on solvent and pH, it was sometimes possible to tune the regiochemistry to a desired result by modifying reaction conditions. This methodology was applied to the direct, regioselective introduction of isopropyl groups into complex, biologically active molecules, such as diflufenican (44) and nevirapine (45).
Figures









References
-
-
For an overview of standard functionalization methods, see Joule JA, Mills K. Heterocyclic Chemistry. 5th. Wiley: Chichester; 2010.
-
-
-
For some recent examples of SNAr chemistry, see: Chen Q, du Jourdin XM, Knochel P. J Am Chem Soc. 2013;135:4958–4961.Bull JA, Mousseau JJ, Pelletier G, Charette AB. Chem Rev. 2012;112:2642–2713.
-
-
-
For examples of functionalization via heteroarene metallation, see: Haag B, Mosrin M, Ila H, Malakhov V, Knochel P. Angew Chem, Int Ed. 2011;50:9794–9824.Schlosser M, Mongin F. Chem Soc Rev. 2007;36:1161–1172.Gros PC, Fort Y. Eur J Org Chem. 2009;2009:4199–4209.Queguiner G, Marsais F, Snieckus V, Epsztajn J. Adv Heterocycl Chem. 1991;52:187–304.Hartung CG, Snieckus V. Modern Arene Chemistry. Wiley; 2004. pp. 342–344.Miller RE, Rantanen T, Ogilvie KA, Groth U, Snieckus V. Org Lett. 2010;12:2198–2201.
-
-
-
For recent examples of metal-catalyzed methodology for the C-H functionalization of pyridines see: Murphy JM, Liao X, Hartwig JF. J Am Chem Soc. 2007;129:15434–15435.Takagi J, Sato K, Hartwig JF, Ishiyama T, Miyaura N. Tetrahedron Lett. 2002;43:5649–5651.Liu T, Shao X, Wu Y, Shen Q. Angew Chem, Int Ed. 2012;51:540–543.Nakao Y, Yamada Y, Kashihara N, Hiyama T. J Am Chem Soc. 2010;132:13666–13668.Wasa M, Worrell BT, Yu JQ. Angew Chem, Int Ed. 2010;49:1275–1277.Ye M, Gao GL, Edmunds AJF, Worthington PA, Morris JA, Yu JQ. J Am Chem Soc. 2011;133:19090–19093.Ye M, Gao GL, Yu JQ. J Am Chem Soc. 2011;133:6964–6967.Berman AM, Lewis JC, Bergman RG, Ellman JA. J Am Chem Soc. 2008;130:14926–14927.Campeau LC, Rousseaux S, Fagnou K. J Am Chem Soc. 2005;127:18020–18021.Cho SH, Hwang SJ, Chang S. J Am Chem Soc. 2008;130:9254–9256.Lewis JC, Bergman RG, Ellman JA. J Am Chem Soc. 2007;129:5332–5333.Nakao Y, Kanyiva KS, Hiyama T. J Am Chem Soc. 2008;130:2448–2449.
-
-
-
For a review on applications of Minisci chemistry in medicinal chemistry see: Duncton MAJ. MedChemComm. 2011;2:1135–1161.
-
Intramolecular examples of homolytic aromatic substitutions on heterocycles are more commonly used in synthesis, see for example: Bowman WR, Storey JMD. Chem Soc Rev. 2007;36:1803–1822.Harrowven DC, Sutton BJ, Coulton S. Org Biomol Chem. 2003;1:4047–4057.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

