A novel long-acting glucose-dependent insulinotropic peptide analogue: enhanced efficacy in normal and diabetic rodents
- PMID: 23859463
- PMCID: PMC4237114
- DOI: 10.1111/dom.12181
A novel long-acting glucose-dependent insulinotropic peptide analogue: enhanced efficacy in normal and diabetic rodents
Abstract
Aim: Glucose-dependent insulinotropic peptide (GIP) is an incretin hormone that is released from intestinal K cells in response to nutrient ingestion. We aimed to investigate the therapeutic potential of the novel N- and C-terminally modified GIP analogue AC163794.
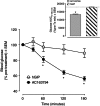
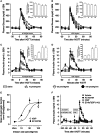
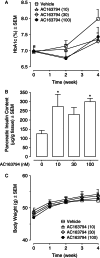
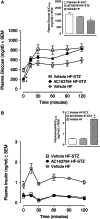
Methods: AC163794 was synthesized by solid-phase peptide synthesis. Design involved the substitution of the C-terminus tail region of the dipeptidyl peptidase IV (DPP-IV)-resistant GIP analogue [d-Ala(2) ]GIP(1-42) with the unique nine amino acid tail region of exenatide. The functional activity and binding of AC163794 to the GIP receptor were evaluated in RIN-m5F β-cells. In vitro metabolic stability was tested in human plasma and kidney membrane preparations. Acute insulinotropic effects were investigated in isolated mouse islets and during an intravenous glucose tolerance test in normal and diabetic Zucker fatty diabetic (ZDF) rats. The biological actions of AC163794 were comprehensively assessed in normal, ob/ob and high-fat-fed streptozotocin (STZ)-induced diabetic mice. Acute glucoregulatory effects of AC163794 were tested in diet-induced obese mice treated subchronically with AC3174, the exendatide analogue [Leu(14) ] exenatide. Human GIP or [d-Ala(2) ]GIP(1-42) were used for comparison.
Results: AC163794 exhibited nanomolar functional GIP receptor potency in vitro similar to GIP and [d-Ala(2) ]GIP(1-42). AC163794 was metabolically more stable in vitro and displayed longer duration of insulinotropic action in vivo versus GIP and [d-Ala(2) ]GIP(1-42). In diabetic mice, AC163794 improved HbA1c through enhanced insulinotropic action, partial restoration of pancreatic insulin content and improved insulin sensitivity with no adverse effects on fat storage and metabolism. AC163794 provided additional baseline glucose-lowering when injected to mice treated with AC3174.
Conclusions: These studies support the potential use of a novel GIP analogue AC163794 for the treatment of type 2 diabetes.
Keywords: DPP-IV resistant; GIP; Trp-cage; diabetes; rodents.
© 2013 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Figures



References
-
- Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, Marks V. Glucagon-like peptide-1 (7–36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol. 1993;138:159–166. - PubMed
-
- Jorde R, Burhol PG, Waldum HL, Schulz TB, Lygren I, Florholmen J. Diurnal variation of plasma gastric inhibitory polypeptide in man. Scand J Gastroenterol. 1980;15:617–619. - PubMed
-
- Dupre J, Ross SA, Watson D, Brown JC. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metabol. 1973;37:826–828. - PubMed
-
- Pederson RA, Schubert HE, Brown JC. Gastric inhibitory polypeptide. Its physiologic release and insulinotropic action in the dog. Diabetes. 1975;24:1050–1056. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

