The current state-of-the-art of spinal cord imaging: applications
- PMID: 23859923
- PMCID: PMC4371134
- DOI: 10.1016/j.neuroimage.2013.07.014
The current state-of-the-art of spinal cord imaging: applications
Abstract
A first-ever spinal cord imaging meeting was sponsored by the International Spinal Research Trust and the Wings for Life Foundation with the aim of identifying the current state-of-the-art of spinal cord imaging, the current greatest challenges, and greatest needs for future development. This meeting was attended by a small group of invited experts spanning all aspects of spinal cord imaging from basic research to clinical practice. The greatest current challenges for spinal cord imaging were identified as arising from the imaging environment itself; difficult imaging environment created by the bone surrounding the spinal canal, physiological motion of the cord and adjacent tissues, and small crosssectional dimensions of the spinal cord, exacerbated by metallic implants often present in injured patients. Challenges were also identified as a result of a lack of "critical mass" of researchers taking on the development of spinal cord imaging, affecting both the rate of progress in the field, and the demand for equipment and software to manufacturers to produce the necessary tools. Here we define the current state-of-the-art of spinal cord imaging, discuss the underlying theory and challenges, and present the evidence for the current and potential power of these methods. In two review papers (part I and part II), we propose that the challenges can be overcome with advances in methods, improving availability and effectiveness of methods, and linking existing researchers to create the necessary scientific and clinical network to advance the rate of progress and impact of the research.
Keywords: Diffusion; Functional MRI; Magnetic resonance; Pathology; Spinal cord; Spinal cord injury.
© 2013.
Figures


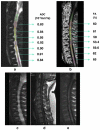
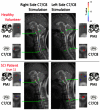

References
-
- Agosta F, Filippi M. MRI of spinal cord in multiple sclerosis. J. Neuroimaging. 2007;17(Suppl. 1):46S–49S. - PubMed
-
- Agosta F, Benedetti B, Rocca MA, Valsasina P, Rovaris M, Comi G, Filippi M. Quantification of cervical cord pathology in primary progressive MS using diffusion tensor MRI. Neurology. 2005;64:631–635. - PubMed
-
- Agosta F, Absinta M, Sormani MP, Ghezzi A, Bertolotto A, Montanari E, Comi G, Filippi M. In vivo assessment of cervical cord damage in MS patients: a longitudinal diffusion tensor MRI study. Brain. 2007a;130:2211–2219. - PubMed
-
- Agosta F, Pagani E, Caputo D, Filippi M. Associations between cervical cord gray matter damage and disability in patients with multiple sclerosis. Arch. Neurol. 2007b;64:1302–1305. - PubMed
-
- Agosta F, Valsasina P, Caputo D, Stroman PW, Filippi M. Tactile-associated recruitment of the cervical cord is altered in patients with multiple sclerosis. NeuroImage. 2008a;39:1542–1548. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

