TNF-α induces phenotypic modulation in cerebral vascular smooth muscle cells: implications for cerebral aneurysm pathology
- PMID: 23860374
- PMCID: PMC3790924
- DOI: 10.1038/jcbfm.2013.109
TNF-α induces phenotypic modulation in cerebral vascular smooth muscle cells: implications for cerebral aneurysm pathology
Abstract
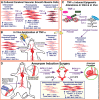
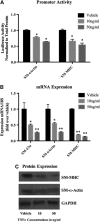
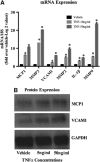
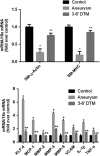
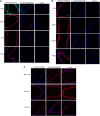
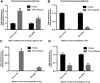
Little is known about vascular smooth muscle cell (SMC) phenotypic modulation in the cerebral circulation or pathogenesis of intracranial aneurysms. Tumor necrosis factor-alpha (TNF-α) has been associated with aneurysms, but potential mechanisms are unclear. Cultured rat cerebral SMCs overexpressing myocardin induced expression of key SMC contractile genes (SM-α-actin, SM-22α, smooth muscle myosin heavy chain), while dominant-negative cells suppressed expression. Tumor necrosis factor-alpha treatment inhibited this contractile phenotype and induced pro-inflammatory/matrix-remodeling genes (monocyte chemoattractant protein-1, matrix metalloproteinase-3, matrix metalloproteinase-9, vascular cell adhesion molecule-1, interleukin-1 beta). Tumor necrosis factor-alpha increased expression of KLF4, a known regulator of SMC differentiation. Kruppel-like transcription factor 4 (KLF4) small interfering RNA abrogated TNF-α activation of inflammatory genes and suppression of contractile genes. These mechanisms were confirmed in vivo after exposure of rat carotid arteries to TNF-α and early on in a model of cerebral aneurysm formation. Treatment with the synthesized TNF-α inhibitor 3,6-dithiothalidomide reversed pathologic vessel wall alterations after induced hypertension and hemodynamic stress. Chromatin immunoprecipitation assays in vivo and in vitro demonstrated that TNF-α promotes epigenetic changes through KLF4-dependent alterations in promoter regions of myocardin, SMCs, and inflammatory genes. In conclusion, TNF-α induces phenotypic modulation of cerebral SMCs through myocardin and KLF4-regulated pathways. These results demonstrate a novel role for TNF-α in promoting a pro-inflammatory/matrix-remodeling phenotype, which has important implications for the mechanisms behind intracranial aneurysm formation.
Figures

References
-
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. - PubMed
-
- Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol. 2012;74:13–40. - PubMed
-
- Yoshida T, Owens GK. Molecular determinants of vascular smooth muscle cell diversity. Circ Res. 2005;96:280–291. - PubMed
-
- Kilic T, Sohrabifar M, Kurtkaya O, Yildirim O, Elmaci I, Günel M, et al. Expression of structural proteins and angiogenic factors in normal arterial and unruptured and ruptured aneurysm walls Neurosurgery 200557997–1007.discussion 1997-1007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

