Use of functional imaging across clinical phases in CNS drug development
- PMID: 23860483
- PMCID: PMC3731782
- DOI: 10.1038/tp.2013.43
Use of functional imaging across clinical phases in CNS drug development
Abstract
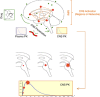
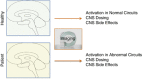
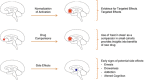
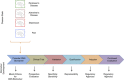
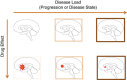
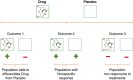
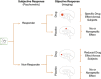
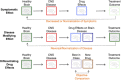
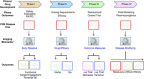
The use of novel brain biomarkers using nuclear magnetic resonance imaging holds potential of making central nervous system (CNS) drug development more efficient. By evaluating changes in brain function in the disease state or drug effects on brain function, the technology opens up the possibility of obtaining objective data on drug effects in the living awake brain. By providing objective data, imaging may improve the probability of success of identifying useful drugs to treat CNS diseases across all clinical phases (I-IV) of drug development. The evolution of functional imaging and the promise it holds to contribute to drug development will require the development of standards (including good imaging practice), but, if well integrated into drug development, functional imaging can define markers of CNS penetration, drug dosing and target engagement (even for drugs that are not amenable to positron emission tomography imaging) in phase I; differentiate objective measures of efficacy and side effects and responders vs non-responders in phase II, evaluate differences between placebo and drug in phase III trials and provide insights into disease modification in phase IV trials.
Figures
References
-
- Drews J. Drug discovery: a historical perspective. Science. 2000;287:1960–1964. - PubMed
-
- Fava M, Rush AJ, Trivedi MH, Nierenberg AA, Thase ME, Sackeim HA, et al. Background and rationale for the sequenced treatment alternatives to relieve depression (STAR*D) study. Psychiatr Clin North Am. 2003;26:457–494. - PubMed
-
- Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287:1840–1847. - PubMed
-
- Pangalos MN, Schechter LE, Hurko O. Drug development for CNS disorders: strategies for balancing risk and reducing attrition. Nat Rev Drug Discov. 2007;6:521–532. - PubMed
-
- Cutler R.Worldwide Clinical Trials 2011. Available from http://www.wwctrials.com .
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

