Sequelae and survivorship in patients treated with (131)I-MIBG therapy
- PMID: 23860527
- PMCID: PMC3738119
- DOI: 10.1038/bjc.2013.365
Sequelae and survivorship in patients treated with (131)I-MIBG therapy
Abstract
Background: (131)I-meta-iodobenzylguanidine ((131)I-MIBG) has been in therapeutic use since 1980s. Newer treatment modalities are emerging for neuroendocrine tumours (NETs) and chromaffin cell tumours (CCTs), but many of these do not yet have adequate long-term follow-up to determine their longer term efficacy and sequelae.
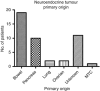
Methods: Fifty-eight patients with metastatic NETs and CCTs who had received (131)I-MIBG therapy between 2000 and 2011 were analysed. Survival and any long-term haematological or renal sequelae were investigated.
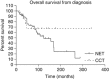
Results: In the NET group, the overall median survival and median survival following the diagnosis of metastatic disease was 124 months. The median survival following the commencement of (131)I-MIBG was 66 months. For the CCT group, median survival had not been reached. The 5-year survival from diagnosis and following the diagnosis of metastatic disease was 67% and 67.5% for NETs and CCTs, respectively. The 5-year survival following the commencement of (131)I-MIBG therapy was 68%. Thirty-two patients had long-term haematological sequelae: 5 of these 32 patients developed haematological malignancies. Two patients developed a mild deterioration in renal function.
Conclusion: Long follow up of (131)I-MIBG therapy reveals a noteable rate of bone marrow toxicities and malignancy and long term review of all patients receiving radionuclide therapies is recommended.
Figures
References
-
- Barakat MT, Meeran K, Bloom SR. Neuroendocrine tumours. Endocr Relat Cancer. 2004;11:1–18. - PubMed
-
- Basu B, Sirohi B, Corrie P. Systemic therapy for neuroendocrine tumours of gastroenteropancreatic origin. Endocr Relat Cancer. 2010;17:R75–R90. - PubMed
-
- Bomanji JB, Papathanasiou ND. (1)(1)(1)In-DTPA(0)-octreotide (Octreoscan), (1)(3)(1)I-MIBG and other agents for radionuclide therapy of NETs. Eur J Nucl Med Mol Imaging. 2012;39 (Suppl 1:S113–S125. - PubMed
-
- Castellani MR, Seghezzi S, Chiesa C, Aliberti GL, Maccauro M, Seregni E, Orunesu E, Luksch R, Bombardieri E. (131)I-MIBG treatment of pheochromocytoma: low versus intermediate activity regimens of therapy. Q J Nucl Med Mol Imaging. 2010;54:100–113. - PubMed
-
- Department of Health MCSNi 2010. The National Cancer Survivorship Initiative Vision: Department of Health publications. www.dh.gov.uk/publications.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical