Substratum compliance modulates corneal fibroblast to myofibroblast transformation
- PMID: 23860754
- PMCID: PMC3757908
- DOI: 10.1167/iovs.12-11575
Substratum compliance modulates corneal fibroblast to myofibroblast transformation
Abstract
Purpose: The transformation of fibroblasts to myofibroblasts is critical to corneal wound healing, stromal haze formation, and scarring. It has recently been demonstrated that the provision of biomimetic substratum topographic cues inhibits the progression toward the myofibroblast phenotype under the influence of transforming growth factor β1 (TGF-β1). The objective of this study was to determine the effect of another fundamental biophysical cue, substrate compliance, on TGF-β1-induced myofibroblast transformation of primary corneal cells isolated from human and rabbit corneas.
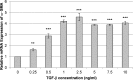
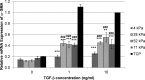
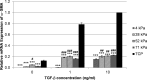
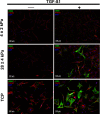
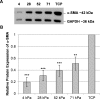
Methods: Human and rabbit corneal fibroblasts were cultured on surfaces of varying substrate compliance (4-71 kPa) and tissue culture plastic (TCP) (> 1 gigapascal [GPa]). Cells were cultured in media containing TGF-β1 at concentrations of 0, 1, or 10 ng/mL for 72 hours. RNA and protein were collected from cells cultured on polyacrylamide gels and TCP and were analyzed for the expression of α-smooth muscle actin (α-SMA), a key marker of myofibroblast transformation, using quantitative PCR, immunocytochemistry, and Western blot.
Results: Cells grown on more compliant substrates demonstrated significantly reduced amounts of α-SMA mRNA compared with TCP. Immunocytochemistry and Western blot analysis determining the presence of α-SMA corroborated this finding, thus confirming a reduced transformation to the myofibroblast phenotype on more compliant substrates compared with cells on TCP in the presence of TGF-β1.
Conclusions: These data indicate that substrate compliance modulates TGF-β1-induced expression of α-SMA and thus influences myofibroblast transformation in the corneal stroma. This provides further evidence that biomimetic biophysical cues inhibit myofibroblast transformation and participate in stabilizing the native cellular phenotype.
Keywords: biophysical cues; corneal wound healing; myofibroblast transformation; α-smooth muscle actin.
Figures
References
-
- Snyder MC, Bergmanson JP, Doughty MJ. Keratocytes: no more the quiet cells. J Am Optom Assoc. 1998; 69: 180–187 - PubMed
-
- Jester JV, Petroll WM, Barry PA, Cavanagh HD. Expression of α-smooth muscle (α-SM) actin during corneal stromal wound healing. Invest Ophthalmol Vis Sci. 1995; 36: 809–819 - PubMed
-
- Netto MV, Mohan RR, Ambrosio R Jr, Hutcheon AE, Zieske JD, Wilson SE. Wound healing in the cornea: a review of refractive surgery complications and new prospects for therapy. Cornea. 2005; 24: 509–522 - PubMed
-
- Saika S, Yamanaka O, Sumioka T, et al. Fibrotic disorders in the eye: targets of gene therapy. Prog Retin Eye Res. 2008; 27: 177–196 - PubMed
-
- Jester JV, Moller-Pedersen T, Huang J, et al. The cellular basis of corneal transparency: evidence for ‘corneal crystallins'. J Cell Sci. 1999; 112 (pt 5): 613–622 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

