Loss of very-long O-antigen chains optimizes capsule-mediated immune evasion by Salmonella enterica serovar Typhi
- PMID: 23860765
- PMCID: PMC3735119
- DOI: 10.1128/mBio.00232-13
Loss of very-long O-antigen chains optimizes capsule-mediated immune evasion by Salmonella enterica serovar Typhi
Abstract
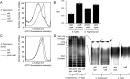
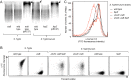
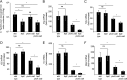
Expression of capsular polysaccharides is a variable trait often associated with more-virulent forms of a bacterial species. For example, typhoid fever is caused by the capsulated Salmonella enterica serovar Typhi, while nontyphoidal Salmonella serovars associated with gastroenteritis are noncapsulated. Here we show that optimization of the immune evasive properties conferred by the virulence-associated (Vi) capsular polysaccharide involved an additional alteration to the cell envelope of S. Typhi, namely inactivation of the fepE gene, encoding the regulator of very-long O-antigen chains. Introduction of the capsule-encoding viaB locus into the nontyphoidal S. enterica serovar Typhimurium reduced complement deposition in vitro and intestinal inflammation in a mouse colitis model. However, both phenotypes were markedly enhanced when the viaB locus was introduced into an S. Typhimurium fepE mutant, which lacks very-long O-antigen chains. Collectively, these data suggest that during the evolution of the S. Typhi lineage, loss of very-long O-antigen chains by pseudogene formation was an adaptation to maximize the anti-inflammatory properties of the Vi capsular polysaccharide.
Importance: Genomic comparison illustrates that acquisition of virulence factors by horizontal gene transfer is an important contributor to the evolution of enteric pathogens. Acquisition of complex virulence traits commonly involves horizontal transfer of a large gene cluster, and integration of the gene cluster into the host genome results in the formation of a pathogenicity island. Acquisition of the virulence-associated (Vi) capsular polysaccharide encoded by SPI7 (Salmonella pathogenicity island 7) was accompanied in the human-adapted Salmonella enterica serovar Typhi by inactivation of the fepE gene, encoding the regulator of very-long O-antigen chains. We show that the resulting loss of very-long O-antigen chains was an important mechanism for maximizing immune evasion mediated by the Vi capsular polysaccharide. These data suggest that successful incorporation of a capsular polysaccharide requires changes in the cell envelope of the hosting pathogen.
Figures

Similar articles
-
The sulfur assimilation pathway mitigates redox stress from acidic pH in Salmonella Typhi H58.mBio. 2025 Jul 9;16(7):e0046725. doi: 10.1128/mbio.00467-25. Epub 2025 May 27. mBio. 2025. PMID: 40422406 Free PMC article.
-
Genetic Ablation of Butyrate Utilization Attenuates Gastrointestinal Salmonella Disease.Cell Host Microbe. 2018 Feb 14;23(2):266-273.e4. doi: 10.1016/j.chom.2018.01.004. Cell Host Microbe. 2018. PMID: 29447698 Free PMC article.
-
Mechanisms to Evade the Phagocyte Respiratory Burst Arose by Convergent Evolution in Typhoidal Salmonella Serovars.Cell Rep. 2018 Feb 13;22(7):1787-1797. doi: 10.1016/j.celrep.2018.01.016. Cell Rep. 2018. PMID: 29444431 Free PMC article.
-
Vaccines for preventing typhoid fever.Cochrane Database Syst Rev. 2018 May 31;5(5):CD001261. doi: 10.1002/14651858.CD001261.pub4. Cochrane Database Syst Rev. 2018. PMID: 29851031 Free PMC article.
-
Typhoid fever: "you can't hit what you can't see".Gut Microbes. 2012 Mar-Apr;3(2):88-92. doi: 10.4161/gmic.18602. Epub 2012 Mar 1. Gut Microbes. 2012. PMID: 22156762 Free PMC article. Review.
Cited by
-
The O-Antigen Capsule of Salmonella enterica Serovar Typhimurium Facilitates Serum Resistance and Surface Expression of FliC.Infect Immun. 2015 Oct;83(10):3946-59. doi: 10.1128/IAI.00634-15. Epub 2015 Jul 20. Infect Immun. 2015. PMID: 26195553 Free PMC article.
-
Salmonella enterica Serovar Typhi Lipopolysaccharide O-Antigen Modification Impact on Serum Resistance and Antibody Recognition.Infect Immun. 2017 Mar 23;85(4):e01021-16. doi: 10.1128/IAI.01021-16. Print 2017 Apr. Infect Immun. 2017. PMID: 28167670 Free PMC article.
-
IL-7 Enables Antibody Responses to Bacterial Polysaccharides by Promoting B Cell Receptor Diversity.J Immunol. 2018 Aug 15;201(4):1229-1240. doi: 10.4049/jimmunol.1800162. Epub 2018 Jul 13. J Immunol. 2018. PMID: 30006375 Free PMC article.
-
Comparative growth analysis of capsulated (Vi+) and acapsulated (Vi-) Salmonella typhi isolates in human blood.EXCLI J. 2015 Feb 9;14:213-9. doi: 10.17179/excli2014-674. eCollection 2015. EXCLI J. 2015. PMID: 26417360 Free PMC article.
-
Salmonellae interactions with host processes.Nat Rev Microbiol. 2015 Apr;13(4):191-205. doi: 10.1038/nrmicro3420. Epub 2015 Mar 9. Nat Rev Microbiol. 2015. PMID: 25749450 Free PMC article. Review.
References
-
- Nasrallah SM, Nassar VH. 1978. Enteric fever: a clinicopathologic study of 104 cases. Am. J. Gastroenterol. 69:63–69 - PubMed
-
- Glynn JR, Palmer SR. 1992. Incubation period, severity of disease, and infecting dose: evidence from a Salmonella outbreak. Am. J. Epidemiol. 136:1369–1377 - PubMed
-
- Barthel M, Hapfelmeier S, Quintanilla-Martínez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Rüssmann H, Hardt WD. 2003. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 71:2839–2858 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

