Calcium ions promote superoxide dismutase 1 (SOD1) aggregation into non-fibrillar amyloid: a link to toxic effects of calcium overload in amyotrophic lateral sclerosis (ALS)?
- PMID: 23861388
- PMCID: PMC3757185
- DOI: 10.1074/jbc.M113.470740
Calcium ions promote superoxide dismutase 1 (SOD1) aggregation into non-fibrillar amyloid: a link to toxic effects of calcium overload in amyotrophic lateral sclerosis (ALS)?
Abstract
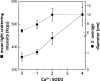
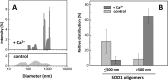
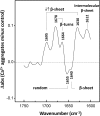
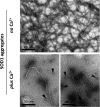
Imbalance in metal ion homeostasis is a hallmark in neurodegenerative conditions involving protein deposition, and amyotrophic lateral sclerosis (ALS) is no exception. In particular, Ca(2+) dysregulation has been shown to correlate with superoxide dismutase-1 (SOD1) aggregation in a cellular model of ALS. Here we present evidence that SOD1 aggregation is enhanced and modulated by Ca(2+). We show that at physiological pH, Ca(2+) induces conformational changes that increase SOD1 β-sheet content, as probed by far UV CD and attenuated total reflectance-FTIR, and enhances SOD1 hydrophobicity, as probed by ANS fluorescence emission. Moreover, dynamic light scattering analysis showed that Ca(2+) boosts the onset of SOD1 aggregation. In agreement, Ca(2+) decreases SOD1 critical concentration and nucleation time during aggregation kinetics, as evidenced by thioflavin T fluorescence emission. Attenuated total reflectance FTIR analysis showed that Ca(2+) induced aggregates consisting preferentially of antiparallel β-sheets, thus suggesting a modulation effect on the aggregation pathway. Transmission electron microscopy and analysis with conformational anti-fibril and anti-oligomer antibodies showed that oligomers and amyloidogenic aggregates constitute the prevalent morphology of Ca(2+)-induced aggregates, thus indicating that Ca(2+) diverts SOD1 aggregation from fibrils toward amorphous aggregates. Interestingly, the same heterogeneity of conformations is found in ALS-derived protein inclusions. We thus hypothesize that transient variations and dysregulation of cellular Ca(2+) levels contribute to the formation of SOD1 aggregates in ALS patients. In this scenario, Ca(2+) may be considered as a pathogenic effector in the formation of ALS proteinaceous inclusions.
Keywords: Amyloid; Amyotrophic Lateral Sclerosis (Lou Gehrig's Disease); Biophysics; Calcium; Circular Dichroism (CD); Electron Microscopy (EM); Infrared Spectroscopy; Neurodegenerative Diseases; Protein Aggregation; Superoxide Dismutase (SOD).
Figures




Similar articles
-
S100A6 amyloid fibril formation is calcium-modulated and enhances superoxide dismutase-1 (SOD1) aggregation.J Biol Chem. 2012 Dec 7;287(50):42233-42. doi: 10.1074/jbc.M112.396416. Epub 2012 Oct 17. J Biol Chem. 2012. PMID: 23076148 Free PMC article.
-
Calcium binding to gatekeeper residues flanking aggregation-prone segments underlies non-fibrillar amyloid traits in superoxide dismutase 1 (SOD1).Biochim Biophys Acta. 2015 Feb;1854(2):118-26. doi: 10.1016/j.bbapap.2014.11.005. Epub 2014 Nov 25. Biochim Biophys Acta. 2015. PMID: 25463043
-
Loss of metal ions, disulfide reduction and mutations related to familial ALS promote formation of amyloid-like aggregates from superoxide dismutase.PLoS One. 2009;4(3):e5004. doi: 10.1371/journal.pone.0005004. Epub 2009 Mar 27. PLoS One. 2009. PMID: 19325915 Free PMC article.
-
Aggregation of copper-zinc superoxide dismutase in familial and sporadic ALS.Antioxid Redox Signal. 2009 Jul;11(7):1603-14. doi: 10.1089/ars.2009.2536. Antioxid Redox Signal. 2009. PMID: 19271992 Free PMC article. Review.
-
The Role of Metal Binding in the Amyotrophic Lateral Sclerosis-Related Aggregation of Copper-Zinc Superoxide Dismutase.Molecules. 2017 Aug 29;22(9):1429. doi: 10.3390/molecules22091429. Molecules. 2017. PMID: 28850080 Free PMC article. Review.
Cited by
-
Ryanodine Receptors: A Potential Treatment Target in Various Neurodegenerative Disease.Cell Mol Neurobiol. 2021 Nov;41(8):1613-1624. doi: 10.1007/s10571-020-00936-w. Epub 2020 Aug 24. Cell Mol Neurobiol. 2021. PMID: 32833122 Free PMC article. Review.
-
Zinc Binding to S100B Affords Regulation of Trace Metal Homeostasis and Excitotoxicity in the Brain.Front Mol Neurosci. 2018 Jan 17;10:456. doi: 10.3389/fnmol.2017.00456. eCollection 2017. Front Mol Neurosci. 2018. PMID: 29386995 Free PMC article.
-
Delineating the Aggregation-Prone Hotspot Regions (Peptides) in the Human Cu/Zn Superoxide Dismutase 1.ACS Omega. 2021 Dec 3;6(49):33985-33994. doi: 10.1021/acsomega.1c05321. eCollection 2021 Dec 14. ACS Omega. 2021. PMID: 34926946 Free PMC article.
-
Serum antioxidant enzymes in spinal stenosis patients with lumbar disc herniation: correlation with degeneration severity and spinal fusion rate.BMC Musculoskelet Disord. 2023 Oct 3;24(1):782. doi: 10.1186/s12891-023-06907-8. BMC Musculoskelet Disord. 2023. PMID: 37789309 Free PMC article.
-
Applications of Induced Pluripotent Stem Cells in Studying the Neurodegenerative Diseases.Stem Cells Int. 2015;2015:382530. doi: 10.1155/2015/382530. Epub 2015 Jul 9. Stem Cells Int. 2015. PMID: 26240571 Free PMC article. Review.
References
-
- Rowland L. P., Shneider N. A. (2001) Amyotrophic lateral sclerosis. N. Engl. J. Med. 344, 1688–1700 - PubMed
-
- Talbot K., Ansorge O. (2006) Recent advances in the genetics of amyotrophic lateral sclerosis and frontotemporal dementia. Common pathways in neurodegenerative disease. Hum. Mol. Genet. 15, R182–R187 - PubMed
-
- Watanabe M., Dykes-Hoberg M., Culotta V. C., Price D. L., Wong P. C., Rothstein J. D. (2001) Histological evidence of protein aggregation in mutant SOD1 transgenic mice and in amyotrophic lateral sclerosis neural tissues. Neurobiol. Dis. 8, 933–941 - PubMed
-
- Bruijn L. I., Houseweart M. K., Kato S., Anderson K. L., Anderson S. D., Ohama E., Reaume A. G., Scott R. W., Cleveland D. W. (1998) Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science 281, 1851–1854 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

