Dystrophin rescue by trans-splicing: a strategy for DMD genotypes not eligible for exon skipping approaches
- PMID: 23861443
- PMCID: PMC3783188
- DOI: 10.1093/nar/gkt621
Dystrophin rescue by trans-splicing: a strategy for DMD genotypes not eligible for exon skipping approaches
Abstract
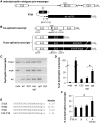
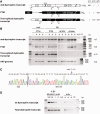
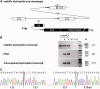
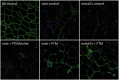
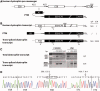
RNA-based therapeutic approaches using splice-switching oligonucleotides have been successfully applied to rescue dystrophin in Duchenne muscular dystrophy (DMD) preclinical models and are currently being evaluated in DMD patients. Although the modular structure of dystrophin protein tolerates internal deletions, many mutations that affect nondispensable domains of the protein require further strategies. Among these, trans-splicing technology is particularly attractive, as it allows the replacement of any mutated exon by its normal version as well as introducing missing exons or correcting duplication mutations. We have applied such a strategy in vitro by using cotransfection of pre-trans-splicing molecule (PTM) constructs along with a reporter minigene containing part of the dystrophin gene harboring the stop-codon mutation found in the mdx mouse model of DMD. Optimization of the different functional domains of the PTMs allowed achieving accurate and efficient trans-splicing of up to 30% of the transcript encoded by the cotransfected minigene. Optimized parameters included mRNA stabilization, choice of splice site sequence, inclusion of exon splice enhancers and artificial intronic sequence. Intramuscular delivery of adeno-associated virus vectors expressing PTMs allowed detectable levels of dystrophin in mdx and mdx4Cv, illustrating that a given PTM can be suitable for a variety of mutations.
Figures
References
-
- Pramono ZA, Takeshima Y, Alimsardjono H, Ishii A, Takeda S, Matsuo M. Induction of exon skipping of the dystrophin transcript in lymphoblastoid cells by transfecting an antisense oligodeoxynucleotide complementary to an exon recognition sequence. Biochem. Biophys. Res. Commun. 1996;226:445–449. - PubMed
-
- Wilton SD, Lloyd F, Carville K, Fletcher S, Honeyman K, Agrawal S, Kole R. Specific removal of the nonsense mutation from the mdx dystrophin mRNA using antisense oligonucleotides. Neuromuscul. Disord. 1999;9:330–338. - PubMed
-
- van Deutekom JC, Janson AA, Ginjaar IB, Frankhuizen WS, Aartsma-Rus A, Bremmer-Bout M, den Dunnen JT, Koop K, van der Kooi AJ, Goemans NM, et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N. Engl. J. Med. 2007;357:2677–2686. - PubMed
-
- Kinali M, Arechavala-Gomeza V, Feng L, Cirak S, Hunt D, Adkin C, Guglieri M, Ashton E, Abbs S, Nihoyannopoulos P, et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009;8:918–928. - PMC - PubMed
-
- Goemans NM, Tulinius M, van den Akker JT, Burm BE, Ekhart PF, Heuvelmans N, Holling T, Janson AA, Platenburg GJ, Sipkens JA, et al. Systemic administration of PRO051 in Duchenne's muscular dystrophy. N. Engl. J. Med. 2011;364:1513–1522. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

