Generation of functionally competent and durable engineered blood vessels from human induced pluripotent stem cells
- PMID: 23861493
- PMCID: PMC3732948
- DOI: 10.1073/pnas.1310675110
Generation of functionally competent and durable engineered blood vessels from human induced pluripotent stem cells
Erratum in
- Proc Natl Acad Sci U S A. 2014 Feb 4;111(5):2047
Abstract
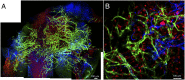
Efficient generation of competent vasculogenic cells is a critical challenge of human induced pluripotent stem (hiPS) cell-based regenerative medicine. Biologically relevant systems to assess functionality of the engineered vessels in vivo are equally important for such development. Here, we report a unique approach for the derivation of endothelial precursor cells from hiPS cells using a triple combination of selection markers--CD34, neuropilin 1, and human kinase insert domain-containing receptor--and an efficient 2D culture system for hiPS cell-derived endothelial precursor cell expansion. With these methods, we successfully generated endothelial cells (ECs) from hiPS cells obtained from healthy donors and formed stable functional blood vessels in vivo, lasting for 280 d in mice. In addition, we developed an approach to generate mesenchymal precursor cells (MPCs) from hiPS cells in parallel. Moreover, we successfully generated functional blood vessels in vivo using these ECs and MPCs derived from the same hiPS cell line. These data provide proof of the principle that autologous hiPS cell-derived vascular precursors can be used for in vivo applications, once safety and immunological issues of hiPS-based cellular therapy have been resolved. Additionally, the durability of hiPS-derived blood vessels in vivo demonstrates a potential translation of this approach in long-term vascularization for tissue engineering and treatment of vascular diseases. Of note, we have also successfully generated ECs and MPCs from type 1 diabetic patient-derived hiPS cell lines and use them to generate blood vessels in vivo, which is an important milestone toward clinical translation of this approach.
Keywords: diabetes; reprogramming; vascular endothelial cells.
Conflict of interest statement
Conflict of interest statement: R.K.J. received research grants from Dyax, MedImmune, and Roche; received consultant fees from Enlight, Noxxon, SynDevRx, WebMD, and Zyngenia; owns equity in Enlight, SynDevRx, and XTuit; and serves on the Board of Directors of XTuit and the Board of Trustees of H&Q Healthcare Investors and H&Q Life Sciences Investors. No reagents or funding from these companies was used in these studies; therefore, there is no significant financial or other competing interest in the work.
Figures



References
-
- Heidenreich PA, et al. American Heart Association Advocacy Coordinating Committee Stroke Council Council on Cardiovascular Radiology and Intervention Council on Clinical Cardiology Council on Epidemiology and Prevention Council on Arteriosclerosis Thrombosis and Vascular Biology Council on Cardiopulmonary Critical Care Perioperative and Resuscitation Council on Cardiovascular Nursing Council on the Kidney in Cardiovascular Disease Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation. 2011;123(8):933–944. - PubMed
-
- Kusuma S, Gerecht S. Engineering blood vessels using stem cells: Innovative approaches to treat vascular disorders. Expert Rev Cardiovasc Ther. 2010;8(10):1433–1445. - PubMed
-
- Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. - PubMed
-
- Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1(1):39–49. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 CA126642/CA/NCI NIH HHS/United States
- R01-CA085140/CA/NCI NIH HHS/United States
- R01CA159258/CA/NCI NIH HHS/United States
- R01 CA085140/CA/NCI NIH HHS/United States
- P41 EB015903/EB/NIBIB NIH HHS/United States
- R01-CA115767/CA/NCI NIH HHS/United States
- K99 HL111343/HL/NHLBI NIH HHS/United States
- P01-CA080124/CA/NCI NIH HHS/United States
- R01-CA096915/CA/NCI NIH HHS/United States
- R01 CA159258/CA/NCI NIH HHS/United States
- K99HL111343-01A1/HL/NHLBI NIH HHS/United States
- R01 CA115767/CA/NCI NIH HHS/United States
- R01 CA096915/CA/NCI NIH HHS/United States
- P01 CA080124/CA/NCI NIH HHS/United States
- R01-CA126642/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

