Emerging complexity of the HuD/ELAVl4 gene; implications for neuronal development, function, and dysfunction
- PMID: 23861535
- PMCID: PMC3708524
- DOI: 10.1261/rna.039164.113
Emerging complexity of the HuD/ELAVl4 gene; implications for neuronal development, function, and dysfunction
Abstract
Precise control of messenger RNA (mRNA) processing and abundance are increasingly being recognized as critical for proper spatiotemporal gene expression, particularly in neurons. These regulatory events are governed by a large number of trans-acting factors found in neurons, most notably RNA-binding proteins (RBPs) and micro-RNAs (miRs), which bind to specific cis-acting elements or structures within mRNAs. Through this binding mechanism, trans-acting factors, particularly RBPs, control all aspects of mRNA metabolism, ranging from altering the transcription rate to mediating mRNA degradation. In this context the best-characterized neuronal RBP, the Hu/ELAVl family member HuD, is emerging as a key component in multiple regulatory processes--including pre-mRNA processing, mRNA stability, and translation--governing the fate of a substantial amount of neuronal mRNAs. Through its ability to regulate mRNA metabolism of diverse groups of functionally similar genes, HuD plays important roles in neuronal development and function. Furthermore, compelling evidence indicates supplementary roles for HuD in neuronal plasticity, in particular, recovery from axonal injury, learning and memory, and multiple neurological diseases. The purpose of this review is to provide a detailed overview of the current knowledge surrounding the expression and roles of HuD in the nervous system. Additionally, we outline the present understanding of the molecular mechanisms presiding over the localization, abundance, and function of HuD in neurons.
Keywords: HuD/ELAVl4; RNA-binding protein; mRNA metabolism; post-transcriptional regulation.
Figures
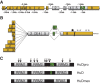
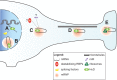
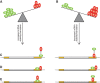
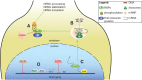
References
-
- Abe R, Uyeno Y, Yamamoto K, Sakamoto H 1994. Tissue-specific expression of the gene encoding a mouse RNA binding protein homologous to human HuD antigen. DNA Res 1: 175–180 - PubMed
-
- Amadio M, Battaini F, Pascale A 2006. The different facets of protein kinases C: Old and new players in neuronal signal transduction pathways. Pharmacol Res 54: 317–325 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
