A new method for the detection of neutralizing antibodies against mumps virus
- PMID: 23861738
- PMCID: PMC3702533
- DOI: 10.1371/journal.pone.0065281
A new method for the detection of neutralizing antibodies against mumps virus
Abstract
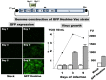
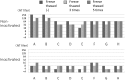
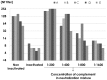
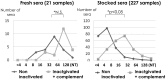
Neutralization test is the most reliable method of evaluating immunity against viral diseases but there is no standard procedure for mumps virus, with tests differing in the infectivity of the challenge virus, 50% plaque reduction or complete inhibition of cytopathic effects (CPE), and usage of complement. A reliable, easy, and simple neutralization test for mumps virus was developed in this study. A recombinant mumps virus expressing GFP was generated as a challenge virus. Complement was added to the neutralizing mixture at 1∶200 when stocked serum samples were used. Neutralizing antibody titers were expressed as the reciprocal of the highest dilution that did not exceed two-fold of FU values (GFP expression) of the cell control wells. A total of 1,452 serum samples were assayed by inhibition of GFP expression in comparison with those examined by conventional 100% inhibition of CPE. 1,367 (94.1%) showed similar neutralizing antibody titers when examined by both methods. The GFP expression inhibition assay, using a recombinant mumps virus expressing GFP, is a simple and time- saving method.
Conflict of interest statement
Figures

Similar articles
-
An efficient, reproducible and accurate RT-qPCR based method to determine mumps specific neutralizing antibody.J Virol Methods. 2020 Mar;277:113817. doi: 10.1016/j.jviromet.2020.113817. Epub 2020 Jan 3. J Virol Methods. 2020. PMID: 31911119
-
Impact of complement and difference of cell-based assay and ELISA in determination of neutralization capacity against mumps and measles virus.J Immunol Methods. 2021 Mar;490:112957. doi: 10.1016/j.jim.2021.112957. Epub 2021 Jan 4. J Immunol Methods. 2021. PMID: 33412172
-
Development of a focus reduction neutralization test (FRNT) for detection of mumps virus neutralizing antibodies.J Virol Methods. 2010 Jan;163(1):153-6. doi: 10.1016/j.jviromet.2009.09.006. Epub 2009 Sep 15. J Virol Methods. 2010. PMID: 19761798
-
Neutralization Assay for Chikungunya Virus Infection: Plaque Reduction Neutralization Test.Methods Mol Biol. 2016;1426:273-82. doi: 10.1007/978-1-4939-3618-2_25. Methods Mol Biol. 2016. PMID: 27233280
-
Development of HiBiT-tagged mumps virus-like particles assembled from authentic viral structural proteins for neutralizing testing.Biochem Biophys Res Commun. 2025 Aug 30;776:152236. doi: 10.1016/j.bbrc.2025.152236. Epub 2025 Jun 19. Biochem Biophys Res Commun. 2025. PMID: 40544761
Cited by
-
Assessment of Mumps Virus-Specific Antibodies: Comparison of Plaque Reduction Neutralization Test and Enzyme-Linked Immunosorbent Assay Estimates.J Infect Dis. 2019 Sep 26;220(9):1462-1468. doi: 10.1093/infdis/jiz345. J Infect Dis. 2019. PMID: 31299077 Free PMC article.
-
Development of a novel Newcastle disease virus (NDV) neutralization test based on recombinant NDV expressing enhanced green fluorescent protein.Virol J. 2017 Nov 23;14(1):232. doi: 10.1186/s12985-017-0900-8. Virol J. 2017. PMID: 29169354 Free PMC article.
-
Easy and Rapid Detection of Mumps Virus by Live Fluorescent Visualization of Virus-Infected Cells.PLoS One. 2015 Dec 2;10(12):e0144038. doi: 10.1371/journal.pone.0144038. eCollection 2015. PLoS One. 2015. PMID: 26629699 Free PMC article.
-
Fluorescent and Bioluminescent Reporter Myxoviruses.Viruses. 2016 Aug 4;8(8):214. doi: 10.3390/v8080214. Viruses. 2016. PMID: 27527209 Free PMC article. Review.
References
-
- Carbone KM, Rubin S (2007) Mumps virus. In: Knipe DM, Howley PM, editors. Fields Virology, 5th edition. Philadelphia, Lippincott, Williams & Wilkins. 1527–1550.
-
- Lamb RA, Parks GD (2007) Paramyxoviridae: The viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. 5th edition. Philadelphia, Lippincott Williams and Wilkins. 1449–1496.
-
- Cusi MG, Fischer S, Sedlmeier R, Valassina M, Valensin PE, et al. (2001) Localization of a new neutralizing epitope on the mumps virus hemagglutinin -neuraminidase protein. Virus Research 74: 133–137. - PubMed
-
- Örvell C, Tecle T, Johansson B, Saito H, Samuelson A (2002) Antigenic relationships between six genotypes of the small hydrophobic protein gene of mumps virus. J Gen Virol 83: 2489–2496. - PubMed
-
- Nagai T, Okafuji T, Miyazaki C, Ito Y, Kamada M, et al. (2007) A comparative study of the incidence of aseptic meningitis in symptomatic natural mumps patients and monovalent mumps vaccine recipients in Japan. Vaccine 25: 2742–2747. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

