Systematic review of the empirical evidence of study publication bias and outcome reporting bias - an updated review
- PMID: 23861749
- PMCID: PMC3702538
- DOI: 10.1371/journal.pone.0066844
Systematic review of the empirical evidence of study publication bias and outcome reporting bias - an updated review
Abstract
Background: The increased use of meta-analysis in systematic reviews of healthcare interventions has highlighted several types of bias that can arise during the completion of a randomised controlled trial. Study publication bias and outcome reporting bias have been recognised as a potential threat to the validity of meta-analysis and can make the readily available evidence unreliable for decision making.
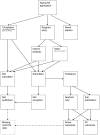
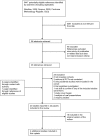
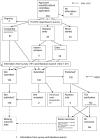
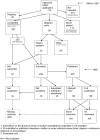
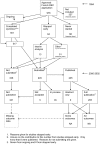
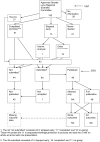
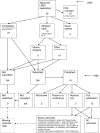
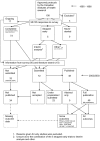
Methodology/principal findings: In this update, we review and summarise the evidence from cohort studies that have assessed study publication bias or outcome reporting bias in randomised controlled trials. Twenty studies were eligible of which four were newly identified in this update. Only two followed the cohort all the way through from protocol approval to information regarding publication of outcomes. Fifteen of the studies investigated study publication bias and five investigated outcome reporting bias. Three studies have found that statistically significant outcomes had a higher odds of being fully reported compared to non-significant outcomes (range of odds ratios: 2.2 to 4.7). In comparing trial publications to protocols, we found that 40-62% of studies had at least one primary outcome that was changed, introduced, or omitted. We decided not to undertake meta-analysis due to the differences between studies.
Conclusions: This update does not change the conclusions of the review in which 16 studies were included. Direct empirical evidence for the existence of study publication bias and outcome reporting bias is shown. There is strong evidence of an association between significant results and publication; studies that report positive or significant results are more likely to be published and outcomes that are statistically significant have higher odds of being fully reported. Publications have been found to be inconsistent with their protocols. Researchers need to be aware of the problems of both types of bias and efforts should be concentrated on improving the reporting of trials.
Conflict of interest statement
Figures
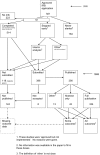
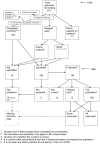
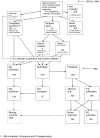
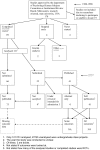
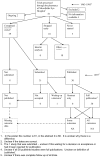
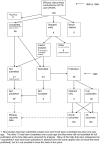
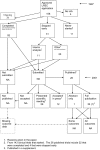
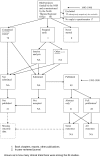
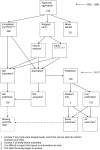
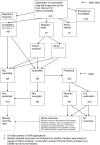
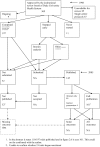
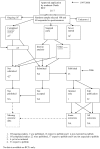
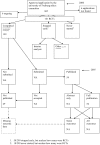
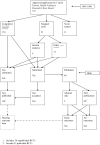
References
-
- Song F, Parekh S, Hooper L, Loke YK, Ryder J, et al.. (2010) Dissemination and publication of research findings: an updated review of related biases.. Health Technol Assess 14. - PubMed
-
- Rothstein HR, Sutton AJ, Borenstein M (2005) Publication Bias in Meta-Analysis. Prevention, Assessment and Adjustments: Wiley.
-
- Dickersin K, Min YI (1993) NIH clinical trials and publication bias. Online J Curr Clin Trials Doc No 50. - PubMed
-
- Ioannidis JP (1998) Effect of the statistical significance of results on the time to completion and publication of randomized efficacy trials. JAMA 279: 281–286. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

