Genome-wide screening for genes associated with valproic acid sensitivity in fission yeast
- PMID: 23861937
- PMCID: PMC3702616
- DOI: 10.1371/journal.pone.0068738
Genome-wide screening for genes associated with valproic acid sensitivity in fission yeast
Abstract
We have been studying the action mechanisms of valproic acid (VPA) in fission yeast Schizosaccharomyces pombe by developing a genetic screen for mutants that show hypersensitivity to VPA. In the present study, we performed a genome-wide screen of 3004 haploid deletion strains and confirmed 148 deletion strains to be VPA sensitive. Of the 148 strains, 93 strains also showed sensitivity to another aliphatic acids HDAC inhibitor, sodium butyrate (SB), and 55 strains showed sensitivity to VPA but not to SB. Interestingly, we found that both VPA and SB treatment induced a marked increase in the transcription activity of Atf1 in wild-type cells. However, in clr6-1, a mutant allele the clr6(+) gene encoding class I HDAC, neither VPA- nor SB induced the activation of Atf1 transcription activity. We also found that VPA, but not SB, caused an increase in cytoplasmic Ca(2+) level. We further found that the cytoplasmic Ca(2+) increase was caused by Ca(2+) influx from extracellular medium via Cch1-Yam8 channel complex. Altogether, our present study indicates that VPA and SB play similar but distinct roles in multiple physiological processes in fission yeast.
Conflict of interest statement
Figures
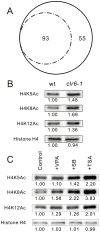
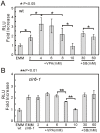
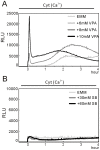

Similar articles
-
Transient receptor potential (TRP) and Cch1-Yam8 channels play key roles in the regulation of cytoplasmic Ca2+ in fission yeast.PLoS One. 2011;6(7):e22421. doi: 10.1371/journal.pone.0022421. Epub 2011 Jul 19. PLoS One. 2011. PMID: 21811607 Free PMC article.
-
Valproic acid affects membrane trafficking and cell-wall integrity in fission yeast.Genetics. 2007 Apr;175(4):1695-705. doi: 10.1534/genetics.107.070946. Epub 2007 Feb 7. Genetics. 2007. PMID: 17287531 Free PMC article.
-
Fingolimod (FTY720) stimulates Ca(2+)/calcineurin signaling in fission yeast.PLoS One. 2013 Dec 3;8(12):e81907. doi: 10.1371/journal.pone.0081907. eCollection 2013. PLoS One. 2013. PMID: 24312601 Free PMC article.
-
A genomewide screen in Schizosaccharomyces pombe for genes affecting the sensitivity of antifungal drugs that target ergosterol biosynthesis.Antimicrob Agents Chemother. 2012 Apr;56(4):1949-59. doi: 10.1128/AAC.05126-11. Epub 2012 Jan 17. Antimicrob Agents Chemother. 2012. PMID: 22252817 Free PMC article.
-
Real-time monitoring of calcineurin activity in living cells: evidence for two distinct Ca2+-dependent pathways in fission yeast.Mol Biol Cell. 2006 Nov;17(11):4790-800. doi: 10.1091/mbc.e06-06-0526. Epub 2006 Aug 23. Mol Biol Cell. 2006. PMID: 16928959 Free PMC article.
Cited by
-
Role of Necroptosis, a Regulated Cell Death, in Seizure and Epilepsy.Neurochem Res. 2024 Jan;49(1):1-13. doi: 10.1007/s11064-023-04010-x. Epub 2023 Aug 30. Neurochem Res. 2024. PMID: 37646959 Review.
-
Histone deacetylases inhibitors effects on Cryptococcus neoformans major virulence phenotypes.Virulence. 2015;6(6):618-30. doi: 10.1080/21505594.2015.1038014. Epub 2015 Jun 23. Virulence. 2015. PMID: 26103530 Free PMC article.
-
Valproate inhibits MAP kinase signalling and cell cycle progression in S. cerevisiae.Sci Rep. 2016 Oct 26;6:36013. doi: 10.1038/srep36013. Sci Rep. 2016. PMID: 27782169 Free PMC article.
-
Sde2 is an intron-specific pre-mRNA splicing regulator activated by ubiquitin-like processing.EMBO J. 2018 Jan 4;37(1):89-101. doi: 10.15252/embj.201796751. Epub 2017 Sep 25. EMBO J. 2018. PMID: 28947618 Free PMC article.
-
Expression of Mug14 is regulated by the transcription factor Rst2 through the cAMP-dependent protein kinase pathway in Schizosaccharomyces pombe.Curr Genet. 2021 Oct;67(5):807-821. doi: 10.1007/s00294-021-01194-z. Epub 2021 Jun 4. Curr Genet. 2021. PMID: 34086083
References
-
- Emrich HM, von Zerssen D, Kissling W, Moller HJ (1981) Therapeutic effect of valproate in mania. Am J Psychiatry 138: 256. - PubMed
-
- Calabresi P, Galletti F, Rossi C, Sarchielli P, Cupini LM (2007) Antiepileptic drugs in migraine: from clinical aspects to cellular mechanisms. Trends Pharmacol Sci 28: 188–195. - PubMed
-
- Johannessen CU (2000) Mechanisms of action of valproate: a commentatory. Neurochem Int 37: 103–110. - PubMed
-
- Gurvich N, Klein PS (2002) Lithium and valproic acid: parallels and contrasts in diverse signaling contexts. Pharmacol Ther 96: 45–66. - PubMed
-
- Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, et al. (2001) Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem 276: 36734–36741. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

