Olfactory ensheathing glia are required for embryonic olfactory axon targeting and the migration of gonadotropin-releasing hormone neurons
- PMID: 23862023
- PMCID: PMC3711043
- DOI: 10.1242/bio.20135249
Olfactory ensheathing glia are required for embryonic olfactory axon targeting and the migration of gonadotropin-releasing hormone neurons
Abstract
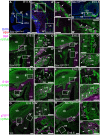
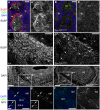
Kallmann's syndrome is caused by the failure of olfactory axons and gonadotropin-releasing hormone (GnRH) neurons to enter the embryonic forebrain, resulting in anosmia and sterility. Sox10 mutations have been associated with Kallmann's syndrome phenotypes, but their effect on olfactory system development is unknown. We recently showed that Sox10 is expressed by neural crest-derived olfactory ensheathing cells (OECs). Here, we demonstrate that in homozygous Sox10(lacZ/lacZ) mouse embryos, OEC differentiation is disrupted; olfactory axons accumulate in the ventromedial olfactory nerve layer and fewer olfactory receptor neurons express the maturation marker OMP (most likely owing to the failure of axonal targeting). Furthermore, GnRH neurons clump together in the periphery and a smaller proportion enters the forebrain. Our data suggest that human Sox10 mutations cause Kallmann's syndrome by disrupting the differentiation of OECs, which promote embryonic olfactory axon targeting and hence olfactory receptor neuron maturation, and GnRH neuron migration to the forebrain.
Keywords: GnRH neurons; Kallmann's syndrome; Olfactory ensheathing glia; Sox10.
Conflict of interest statement
Figures



Similar articles
-
GPR37 Signaling Modulates Migration of Olfactory Ensheathing Cells and Gonadotropin Releasing Hormone Cells in Mice.Front Cell Neurosci. 2019 May 9;13:200. doi: 10.3389/fncel.2019.00200. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31143101 Free PMC article.
-
Olfactory ensheathing cells abutting the embryonic olfactory bulb express Frzb, whose deletion disrupts olfactory axon targeting.Glia. 2018 Dec;66(12):2617-2631. doi: 10.1002/glia.23515. Epub 2018 Sep 26. Glia. 2018. PMID: 30256452 Free PMC article.
-
Neural crest Notch/Rbpj signaling regulates olfactory gliogenesis and neuronal migration.Genesis. 2018 Jun;56(6-7):e23215. doi: 10.1002/dvg.23215. Genesis. 2018. PMID: 30134068 Free PMC article.
-
The great migration: How glial cells could regulate GnRH neuron development and shape adult reproductive life.J Chem Neuroanat. 2022 Nov;125:102149. doi: 10.1016/j.jchemneu.2022.102149. Epub 2022 Sep 1. J Chem Neuroanat. 2022. PMID: 36058434 Review.
-
Kallmann's syndrome, a neuronal migration defect.Cell Mol Life Sci. 2006 Nov;63(21):2512-26. doi: 10.1007/s00018-005-5604-3. Cell Mol Life Sci. 2006. PMID: 16952059 Free PMC article. Review.
Cited by
-
The Glia Response after Peripheral Nerve Injury: A Comparison between Schwann Cells and Olfactory Ensheathing Cells and Their Uses for Neural Regenerative Therapies.Int J Mol Sci. 2017 Jan 29;18(2):287. doi: 10.3390/ijms18020287. Int J Mol Sci. 2017. PMID: 28146061 Free PMC article. Review.
-
Kallmann Syndrome Due to Heterozygous Mutation in SOX10 Coexisting With Waardenburg Syndrome Type II: Case Report and Review of Literature.Front Endocrinol (Lausanne). 2021 Feb 1;11:592831. doi: 10.3389/fendo.2020.592831. eCollection 2020. Front Endocrinol (Lausanne). 2021. PMID: 33597923 Free PMC article. Review.
-
GPR37 Signaling Modulates Migration of Olfactory Ensheathing Cells and Gonadotropin Releasing Hormone Cells in Mice.Front Cell Neurosci. 2019 May 9;13:200. doi: 10.3389/fncel.2019.00200. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31143101 Free PMC article.
-
Defective jagged-1 signaling affects GnRH development and contributes to congenital hypogonadotropic hypogonadism.JCI Insight. 2023 Mar 8;8(5):e161998. doi: 10.1172/jci.insight.161998. JCI Insight. 2023. PMID: 36729644 Free PMC article.
-
GnRH-1 Neural Migration From the Nose to the Brain Is Independent From Slit2, Robo3 and NELL2 Signaling.Front Cell Neurosci. 2019 Mar 1;13:70. doi: 10.3389/fncel.2019.00070. eCollection 2019. Front Cell Neurosci. 2019. PMID: 30881290 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

