Scope and mechanism of the Pt-catalyzed enantioselective diboration of monosubstituted alkenes
- PMID: 23862690
- PMCID: PMC3805123
- DOI: 10.1021/ja4041016
Scope and mechanism of the Pt-catalyzed enantioselective diboration of monosubstituted alkenes
Abstract
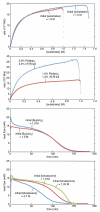
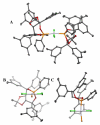
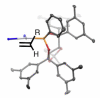
The Pt-catalyzed enantioselective diboration of terminal alkenes can be accomplished in an enantioselective fashion in the presence of chiral phosphonite ligands. Optimal procedures and the substrate scope of this transformation are fully investigated. Reaction progress kinetic analysis and kinetic isotope effects suggest that the stereodefining step in the catalytic cycle is olefin migratory insertion into a Pt-B bond. Density functional theory analysis, combined with other experimental data, suggests that the insertion reaction positions platinum at the internal carbon of the substrate. A stereochemical model for this reaction is advanced that is in line both with these features and with the crystal structure of a Pt-ligand complex.
Figures





References
-
-
Review: Hoveyda AH, Evans DA, Fu GC. Chem. Rev. 1993;93:1307.
-
-
-
Representative enantioselective reactions of aliphatic monosubstituted alkenes (>80%ee): Uozumi Y, Hayashi TJ. Am. Chem. Soc. 1991;113:9887. Becker D, Sharpless KB. Angew. Chem. Int. Ed. 1996;35:448. El-Qisairi A, Hamed O, Henry PM. J. Org. Chem. 1998;63:2790. Lo MC, Fu GC. J. Am. Chem. Soc. 1998;120:10270. Negishi E, Tan Z, Liang B, Novak T. Proc. Natl. Acad. Sci. 2004;101:5782. Colladon M, Scarso A, Sgarbossa P, Michelin RA, Strukul G. J. Am. Chem. Soc. 2006;128:14006. Sawada Y, Matsumoto K, Katsuki T. Angew. Chem. Int. Ed. 2007;46:4559. Zhang Z, Lee SD, Widenhoefer RA. J. Am. Chem. Soc. 2009;131:5372. Noonan GM, Fuentes JA, Cobley CJ, Clarke ML. Angew. Chem. Int. Ed. 2012;51:2477.
-
-
-
Morken JB, Miller SP, Morken JP. J. Am. Chem. Soc. 2003;125:8702. Miller SP, Morgan JB, Nepveux FJ, Morken JP. Org. Lett. 2004;6:131. Trudeau S, Morgan JB, Shrestha M, Morken JP. J. Org. Chem. 2005;70:9538. Kliman LT, Mlynarski SN, Morken JP. J. Am. Chem. Soc. 2009;131:13210. Review: Suginome M, Ohmura T. Boronic Acids. (2nd Ed) 2011;Vol. 1:171. Burks HE, Morken JP. Chem. Commun. 2007:4717.
-
-
-
Reviews: Brown HS, Singaram B. Acc. Chem. Res. 1988;21:287. Ramachandran PV, Brown HC, editors. ACS Symposium Series 783. American Chemical Society; Washington, DC: 2001. Organoboranes for Synthesis. Matteson DS. Stereodirected Synthesis with Organoboranes. Springer; New York: 1995. <underline>Amination</underline>: Brown HC, Midland MM, Levy AB. J. Am. Chem. Soc. 1973;95:2394. Brown HC, Kim KW, Cole TE, Singaram B. J. Am. Chem. Soc. 1986;108:6761. Knight FI, Brown JM, Lazzari D, Ricci A, Blacker AJ. Tetrahedron. 1997;53:11411. Fernandez E, Maeda K, Hooper MW, Brown JM. Chem Eur. J. 2000;6:1840. <underline>Oxidation</underline>: Zweifel G, Brown HC. Org. React. 1963;13:1. Brown HC, Snyder C, Subba Rao BC, Zweifel G. Tetrahedron. 1986;42:5505. Kabalka GW, Wadgaonkar PP, Shoup TM. Organometallics. 1990;9:1316. <underline>Carbon Insertion</underline>: Suzuki A, Miyaura N, Abiko S, Itoh M, Brown HC, Sinclair JA, Midland MM. J. Am. Chem. Soc. 1973;95:3080. Leung T, Zweifel G. J. Am. Chem. Soc. 1974;96:5620. Yamada K, Miyaura N, Itoh M, Suzuki A. Synthesis. 1977:679. Hara S, Dojo H, Kato T, Suzuki A. Chemistry Lett. 1983:1125. Brown HC, Imai T. J. Am. Chem. Soc. 1983;105:6285. Matteson DS, Majumdar D. Organometallics. 1983;2:1529. Sadhu K, M., Matteson DS. Organometallics. 1985;4:1687. Brown HC, Singh SM. Organometallics. 1986;5:994. Brown HC, Singh SM. Organometallics. 1986;5:998. Brown HC, Singh SM, Rangaishenvi MV. J. Org. Chem. 1986;51:3150. Chen AC, Ren L, Crudden CM. Chem. Commun. 1999:611. Ren L, Crudden CM. Chem. Commun. 2000:721. O’Donnell MJ, Drew MD, Cooper JT, Delgado F, Zhou C. J. Am. Chem. Soc. 2002;124:9348. Aggarwal VK, Fang GY, Schmidt AT. J. Am. Chem. Soc. 2005;127:1642. Aggarwal VK. J. Am. Chem. Soc. 2007;129:14632. Coldham I, Patel JJ, Raimbault S, Whittaker DTE, Adams H, Fang GY, Aggarwal VK. Org. Lett. 2008;10:141.
-
-
- Ishiyama T, Matsuda N, Miyaura N, Suzuki A. J. Am. Chem. Soc. 1993;115:11018.
- Iverson CN, Smith MR. J. Am. Chem. Soc. 1995;117:4403.
- Lesley G, Nguyen P, Taylor NJ, Marder TB. Organometallics. 1996;15:5137.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

