Hyperactive Ras/MAPK signaling is critical for tibial nonunion fracture in neurofibromin-deficient mice
- PMID: 23863460
- PMCID: PMC3820137
- DOI: 10.1093/hmg/ddt333
Hyperactive Ras/MAPK signaling is critical for tibial nonunion fracture in neurofibromin-deficient mice
Abstract
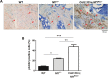
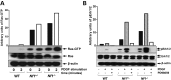
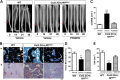
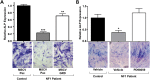
Neurofibromatosis type 1 (NF1) is a common genetic disorder affecting 1 in 3500 individuals. Patients with NF1 are predisposed to debilitating skeletal manifestations, including osteopenia/osteoporosis and long bone pseudarthrosis (nonunion fracture). Hyperactivation of the Ras/mitogen-activated protein kinase (MAPK) pathway in NF1 is known to underlie aberrant proliferation and differentiation in cell lineages, including osteoclast progenitors and mesenchymal stem cells (MSCs) also known as osteoblast progenitors (pro-OBLs). Our current study demonstrates the hyper Ras/MAPK as a critical pathway underlying the pathogenesis of NF1-associated fracture repair deficits. Nf1-deficient pro-OBLs exhibit Ras/MAPK hyperactivation. Introduction of the NF1 GTPase activating-related domain (NF1 GAP-related domain) in vitro is sufficient to rescue hyper Ras activity and enhance osteoblast (OBL) differentiation in Nf1(-/-) pro-OBLs and NF1 human (h) MSCs cultured from NF1 patients with skeletal abnormalities, including pseudarthrosis or scoliosis. Pharmacologic inhibition of mitogen-activated protein kinase kinase (MEK) signaling with PD98059 partially rescues aberrant Erk activation while enhancing OBL differentiation and expression of OBL markers, osterix and osteocalcin, in Nf1-deficient murine pro-OBLs. Similarly, MEK inhibition enhances OBL differentiation of hMSCs. In addition, PD98059 rescues aberrant osteoclast maturation in Nf1 haploinsufficient bone marrow mononuclear cells (BMMNCs). Importantly, MEK inhibitor significantly improves fracture healing in an NF1 murine model, Col2.3Cre;Nf1(flox/-). Collectively, these data indicate the Ras/MAPK cascade as a critical pathway in the pathogenesis of bone loss and pseudarthrosis related to NF1 mutations. These studies provide evidence for targeting the MAPK pathway to improve bone mass and treat pseudarthrosis in NF1.
Figures



Similar articles
-
Hyperactive transforming growth factor-β1 signaling potentiates skeletal defects in a neurofibromatosis type 1 mouse model.J Bone Miner Res. 2013 Dec;28(12):2476-89. doi: 10.1002/jbmr.1992. J Bone Miner Res. 2013. PMID: 23703870 Free PMC article.
-
Combined MEK inhibition and BMP2 treatment promotes osteoblast differentiation and bone healing in Nf1Osx -/- mice.J Bone Miner Res. 2015 Jan;30(1):55-63. doi: 10.1002/jbmr.2316. J Bone Miner Res. 2015. PMID: 25043591 Free PMC article.
-
MEK-SHP2 inhibition prevents tibial pseudarthrosis caused by NF1 loss in Schwann cells and skeletal stem/progenitor cells.Sci Transl Med. 2024 Jun 26;16(753):eadj1597. doi: 10.1126/scitranslmed.adj1597. Epub 2024 Jun 26. Sci Transl Med. 2024. PMID: 38924432 Free PMC article.
-
Aberrant Myeloid Differentiation Contributes to the Development of Osteoporosis in Neurofibromatosis Type 1.Curr Osteoporos Rep. 2016 Feb;14(1):10-5. doi: 10.1007/s11914-016-0298-z. Curr Osteoporos Rep. 2016. PMID: 26932441 Review.
-
Legius syndrome, an Update. Molecular pathology of mutations in SPRED1.Keio J Med. 2013;62(4):107-12. doi: 10.2302/kjm.2013-0002-re. Epub 2013 Dec 10. Keio J Med. 2013. PMID: 24334617 Review.
Cited by
-
Bone marrow mesenchymal stem cell-derived exosomes promote osteoblast proliferation, migration and inhibit apoptosis by regulating KLF3-AS1/miR-338-3p.BMC Musculoskelet Disord. 2024 Feb 9;25(1):122. doi: 10.1186/s12891-024-07236-0. BMC Musculoskelet Disord. 2024. PMID: 38336637 Free PMC article.
-
Resting state functional MRI reveals abnormal network connectivity in neurofibromatosis 1.Hum Brain Mapp. 2015 Nov;36(11):4566-81. doi: 10.1002/hbm.22937. Epub 2015 Aug 25. Hum Brain Mapp. 2015. PMID: 26304096 Free PMC article.
-
Challenges in the Management of a Calvarial Defect in an NF1-Patient.Diseases. 2024 Dec 12;12(12):325. doi: 10.3390/diseases12120325. Diseases. 2024. PMID: 39727655 Free PMC article.
-
Clinical characteristics and in silico analysis of congenital pseudarthrosis of the tibia combined with neurofibromatosis type 1 caused by a novel NF1 mutation.Front Genet. 2022 Sep 28;13:991314. doi: 10.3389/fgene.2022.991314. eCollection 2022. Front Genet. 2022. PMID: 36246612 Free PMC article.
-
Mechanisms of action of neuropeptide Y on stem cells and its potential applications in orthopaedic disorders.World J Stem Cells. 2020 Sep 26;12(9):986-1000. doi: 10.4252/wjsc.v12.i9.986. World J Stem Cells. 2020. PMID: 33033559 Free PMC article. Review.
References
-
- Gutmann D.H. The neurofibromatoses: when less is more. Hum. Mol. Genet. 2001;10:747–755. - PubMed
-
- Theos A., Korf B.R. Pathophysiology of neurofibromatosis type 1. Ann. Intern. Med. 2006;144:842–849. - PubMed
-
- Xu G.F., O'Connell P., Viskochil D., Cawthon R., Robertson M., Culver M., Dunn D., Stevens J., Gesteland R., White R., et al. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell. 1990;62:599–608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

