Tumor suppressor Nf2 limits expansion of the neural progenitor pool by inhibiting Yap/Taz transcriptional coactivators
- PMID: 23863479
- PMCID: PMC3737715
- DOI: 10.1242/dev.096537
Tumor suppressor Nf2 limits expansion of the neural progenitor pool by inhibiting Yap/Taz transcriptional coactivators
Abstract
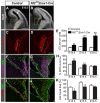
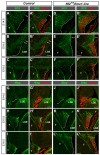
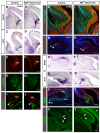
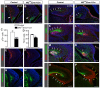
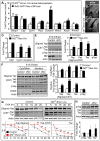
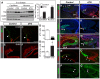
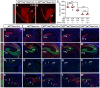
Brain development requires a precise balance between expansion of the neural progenitor pool and the production of postmitotic neurons and glia. Disruption of this equilibrium results in a myriad of structural abnormalities and disorders of the nervous system. The molecular mechanism that restricts neural progenitor expansion is poorly understood. Here we show that the tumor suppressor neurofibromatosis 2 (Nf2; merlin) limits the expansion of neural progenitor cells (NPCs) in the mammalian dorsal telencephalon. Nf2 is localized at the apical region of NPCs. In the absence of Nf2, NPCs of the cortical hem, hippocampal primordium and neocortical primordium overexpand, while production of Cajal-Retzius cells and hippocampal neurons decreases, resulting in severe malformation of the hippocampus in adult mice. We further show that Nf2 functions by inhibiting the Yap/Taz transcriptional coactivators, probably through a mechanism that is distinct from the canonical Hippo pathway. Overexpressing human YAP in NPCs causes a hippocampal malformation phenotype that closely resembles that of Nf2 mutants and, importantly, deleting Yap in the Nf2 mutant background largely restores hippocampal development. Our studies uncover Nf2 as an important inhibitor of neural progenitor expansion and establish Yap/Taz as key downstream effectors of Nf2 during brain development.
Keywords: Archicortex; Mouse; Neurogenesis; Radial glial cells; Wwtr1; Yap1.
Figures

References
-
- Akhmametyeva E. M., Mihaylova M. M., Luo H., Kharzai S., Welling D. B., Chang L. S. (2006). Regulation of the neurofibromatosis 2 gene promoter expression during embryonic development. Dev. Dyn. 235, 2771–2785 - PubMed
-
- Altman J., Bayer S. A. (1990). Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J. Comp. Neurol. 301, 365–381 - PubMed
-
- Barry E. R., Camargo F. D. (2013). The Hippo superhighway: signaling crossroads converging on the Hippo/Yap pathway in stem cells and development. Curr. Opin. Cell Biol. 25, 247–253 - PubMed
-
- Baser M. E., R Evans D. G., Gutmann D. H. (2003). Neurofibromatosis 2. Curr. Opin. Neurol. 16, 27–33 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

