Notch3 signaling gates cell cycle entry and limits neural stem cell amplification in the adult pallium
- PMID: 23863484
- PMCID: PMC3737716
- DOI: 10.1242/dev.095018
Notch3 signaling gates cell cycle entry and limits neural stem cell amplification in the adult pallium
Abstract
Maintaining the homeostasis of germinal zones in adult organs is a fundamental but mechanistically poorly understood process. In particular, what controls stem cell activation remains unclear. We have previously shown that Notch signaling limits neural stem cell (NSC) proliferation in the adult zebrafish pallium. Combining pharmacological and genetic manipulations, we demonstrate here that long-term Notch invalidation primarily induces NSC amplification through their activation from quiescence and increased occurrence of symmetric divisions. Expression analyses, morpholino-mediated invalidation and the generation of a notch3-null mutant directly implicate Notch3 in these effects. By contrast, abrogation of notch1b function results in the generation of neurons at the expense of the activated NSC state. Together, our results support a differential involvement of Notch receptors along the successive steps of NSC recruitment. They implicate Notch3 at the top of this hierarchy to gate NSC activation and amplification, protecting the homeostasis of adult NSC reservoirs under physiological conditions.
Keywords: Adult neural stem cell; Notch3; Quiescence.
Figures


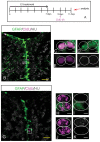
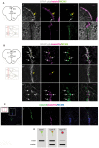

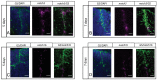
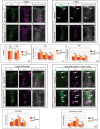
References
-
- Adolf B., Chapouton P., Lam C. S., Topp S., Tannhäuser B., Strähle U., Götz M., Bally-Cuif L. (2006). Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev. Biol. 295, 278–293 - PubMed
-
- Andreu-Agulló C., Morante-Redolat J. M., Delgado A. C., Fariñas I. (2009). Vascular niche factor PEDF modulates Notch-dependent stemness in the adult subependymal zone. Nat. Neurosci. 12, 1514–1523 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

