Long-term protective immunity from an influenza virus-like particle vaccine administered with a microneedle patch
- PMID: 23863506
- PMCID: PMC3889580
- DOI: 10.1128/CVI.00251-13
Long-term protective immunity from an influenza virus-like particle vaccine administered with a microneedle patch
Abstract
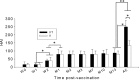
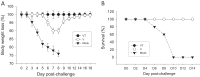
Skin vaccination with influenza virus-like particles (VLPs) using microneedles has been shown to induce protection similar to or better than that induced by intramuscular immunization. In this study, we examined the long-term protective efficacy of influenza (H1N1 A/PR/8/34) VLPs after skin vaccination using microneedle patches coated with the vaccine. Microneedle vaccination of mice in the skin induced 100% protection against lethal challenge infection with influenza A/PR/8/34 virus 14 months after a single vaccine dose. Influenza virus-specific total IgG response and hemagglutination inhibition (HAI) titers were maintained at high levels for over 1 year after microneedle vaccination. Microneedle vaccination also induced substantial levels of lung IgG and IgA antibody responses, and antibody-secreting plasma cells from spleen and bone marrow, as well as conferring effective control of lung viral loads, resulting in complete protection 14 months after vaccination. These strong and long-lasting immune responses were enabled in part by stabilization of the vaccine by formulation with trehalose during microneedle patch fabrication. Administration of the stabilized vaccine using microneedles was especially effective at enabling strong recall responses measured 4 days after lethal virus challenge, including increased HAI and antibody-secreting cells in the spleen and reduced viral titer and inflammatory response in the lung. The results in this study indicate that skin vaccination with VLP vaccine using a microneedle patch provides long-term protection against influenza in mice.
Figures

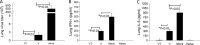


Similar articles
-
Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization.J Virol. 2010 Aug;84(15):7760-9. doi: 10.1128/JVI.01849-09. Epub 2010 May 19. J Virol. 2010. PMID: 20484519 Free PMC article.
-
Improved protection against avian influenza H5N1 virus by a single vaccination with virus-like particles in skin using microneedles.Antiviral Res. 2010 Nov;88(2):244-7. doi: 10.1016/j.antiviral.2010.09.001. Epub 2010 Sep 21. Antiviral Res. 2010. PMID: 20851715 Free PMC article.
-
Dose sparing enabled by skin immunization with influenza virus-like particle vaccine using microneedles.J Control Release. 2010 Nov 1;147(3):326-32. doi: 10.1016/j.jconrel.2010.07.125. Epub 2010 Aug 6. J Control Release. 2010. PMID: 20692307 Free PMC article.
-
Microneedle-based vaccines.Curr Top Microbiol Immunol. 2009;333:369-93. doi: 10.1007/978-3-540-92165-3_18. Curr Top Microbiol Immunol. 2009. PMID: 19768415 Free PMC article. Review.
-
Influenza Vaccines toward Universality through Nanoplatforms and Given by Microneedle Patches.Viruses. 2020 Oct 24;12(11):1212. doi: 10.3390/v12111212. Viruses. 2020. PMID: 33114336 Free PMC article. Review.
Cited by
-
Insect Cell-Based Quadrivalent Seasonal Influenza Virus-like Particles Vaccine Elicits Potent Immune Responses in Mice.Vaccines (Basel). 2024 Jun 17;12(6):667. doi: 10.3390/vaccines12060667. Vaccines (Basel). 2024. PMID: 38932396 Free PMC article.
-
Progress in microneedle array patch (MAP) for vaccine delivery.Hum Vaccin Immunother. 2021 Jan 2;17(1):316-327. doi: 10.1080/21645515.2020.1767997. Epub 2020 Jul 15. Hum Vaccin Immunother. 2021. PMID: 32667239 Free PMC article.
-
Skin permeabilization for transdermal drug delivery: recent advances and future prospects.Expert Opin Drug Deliv. 2014 Mar;11(3):393-407. doi: 10.1517/17425247.2014.875528. Epub 2014 Jan 7. Expert Opin Drug Deliv. 2014. PMID: 24392787 Free PMC article. Review.
-
Skin immunization with influenza vaccines.Curr Top Microbiol Immunol. 2015;386:343-69. doi: 10.1007/82_2014_407. Curr Top Microbiol Immunol. 2015. PMID: 25038939 Free PMC article. Review.
-
Universal influenza vaccines: from viruses to nanoparticles.Expert Rev Vaccines. 2018 Nov;17(11):967-976. doi: 10.1080/14760584.2018.1541408. Epub 2018 Nov 2. Expert Rev Vaccines. 2018. PMID: 30365905 Free PMC article. Review.
References
-
- Osterholm MT. 2005. Preparing for the next pandemic. N. Engl. J. Med. 352:1839–1842 - PubMed
-
- Galarza JM, Latham T, Cupo A. 2005. Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 18:244–251 - PubMed
-
- Pushko P, Tumpey TM, Bu F, Knell J, Robinson R, Smith G. 2005. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine 23:5751–5759 - PubMed
-
- Bright RA, Carter DM, Daniluk S, Toapanta FR, Ahmad A, Gavrilov V, Massare M, Pushko P, Mytle N, Rowe T, Smith G, Ross TM. 2007. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine 25:3871–3878 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

