Gray platelet syndrome and defective thrombo-inflammation in Nbeal2-deficient mice
- PMID: 23863626
- PMCID: PMC4011026
- DOI: 10.1172/JCI69210
Gray platelet syndrome and defective thrombo-inflammation in Nbeal2-deficient mice
Abstract
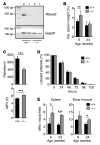
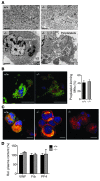
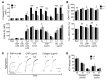
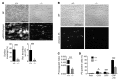
Platelets are anuclear organelle-rich cell fragments derived from bone marrow megakaryocytes (MKs) that safeguard vascular integrity. The major platelet organelles, α-granules, release proteins that participate in thrombus formation and hemostasis. Proteins stored in α-granules are also thought to play a role in inflammation and wound healing, but their functional significance in vivo is unknown. Mutations in NBEAL2 have been linked to gray platelet syndrome (GPS), a rare bleeding disorder characterized by macrothrombocytopenia, with platelets lacking α-granules. Here we show that Nbeal2-knockout mice display the characteristics of human GPS, with defective α-granule biogenesis in MKs and their absence from platelets. Nbeal2 deficiency did not affect MK differentiation and proplatelet formation in vitro or platelet life span in vivo. Nbeal2-deficient platelets displayed impaired adhesion, aggregation, and coagulant activity ex vivo that translated into defective arterial thrombus formation and protection from thrombo-inflammatory brain infarction following focal cerebral ischemia. In a model of excisional skin wound repair, Nbeal2-deficient mice exhibited impaired development of functional granulation tissue due to severely reduced differentiation of myofibroblasts in the absence of α-granule secretion. This study demonstrates that platelet α-granule constituents are critically required not only for hemostasis but also thrombosis, acute thrombo-inflammatory disease states, and tissue reconstitution after injury.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

