Transcription factor EGR1 directs tendon differentiation and promotes tendon repair
- PMID: 23863709
- PMCID: PMC4011025
- DOI: 10.1172/JCI67521
Transcription factor EGR1 directs tendon differentiation and promotes tendon repair
Abstract
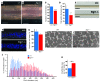
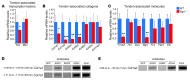
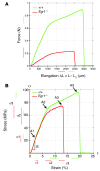
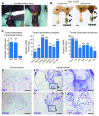
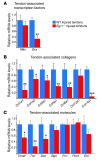
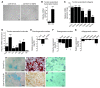
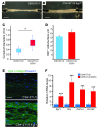
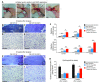
Tendon formation and repair rely on specific combinations of transcription factors, growth factors, and mechanical parameters that regulate the production and spatial organization of type I collagen. Here, we investigated the function of the zinc finger transcription factor EGR1 in tendon formation, healing, and repair using rodent animal models and mesenchymal stem cells (MSCs). Adult tendons of Egr1-/- mice displayed a deficiency in the expression of tendon genes, including Scx, Col1a1, and Col1a2, and were mechanically weaker compared with their WT littermates. EGR1 was recruited to the Col1a1 and Col2a1 promoters in postnatal mouse tendons in vivo. Egr1 was required for the normal gene response following tendon injury in a mouse model of Achilles tendon healing. Forced Egr1 expression programmed MSCs toward the tendon lineage and promoted the formation of in vitro-engineered tendons from MSCs. The application of EGR1-producing MSCs increased the formation of tendon-like tissues in a rat model of Achilles tendon injury. We provide evidence that the ability of EGR1 to promote tendon differentiation is partially mediated by TGF-β2. This study demonstrates EGR1 involvement in adult tendon formation, healing, and repair and identifies Egr1 as a putative target in tendon repair strategies.
Figures
Comment in
-
Development. EGR1 is a key factor in tendon development and healing.Nat Rev Rheumatol. 2013 Sep;9(9):505. doi: 10.1038/nrrheum.2013.125. Epub 2013 Aug 13. Nat Rev Rheumatol. 2013. PMID: 23938861 No abstract available.
References
-
- Schweitzer R, et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128(19):3855–3866. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

