Inner ear supporting cells protect hair cells by secreting HSP70
- PMID: 23863716
- PMCID: PMC3967657
- DOI: 10.1172/JCI68480
Inner ear supporting cells protect hair cells by secreting HSP70
Abstract
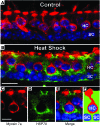
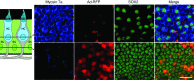
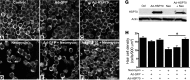
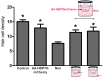
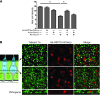
Mechanosensory hair cells are the receptor cells of hearing and balance. Hair cells are sensitive to death from exposure to therapeutic drugs with ototoxic side effects, including aminoglycoside antibiotics and cisplatin. We recently showed that the induction of heat shock protein 70 (HSP70) inhibits ototoxic drug-induced hair cell death. Here, we examined the mechanisms underlying the protective effect of HSP70. In response to heat shock, HSP70 was induced in glia-like supporting cells but not in hair cells. Adenovirus-mediated infection of supporting cells with Hsp70 inhibited hair cell death. Coculture with heat-shocked utricles protected nonheat-shocked utricles against hair cell death. When heat-shocked utricles from Hsp70-/- mice were used in cocultures, protection was abolished in both the heat-shocked utricles and the nonheat-shocked utricles. HSP70 was detected by ELISA in the media surrounding heat-shocked utricles, and depletion of HSP70 from the media abolished the protective effect of heat shock, suggesting that HSP70 is secreted by supporting cells. Together our data indicate that supporting cells mediate the protective effect of HSP70 against hair cell death, and they suggest a major role for supporting cells in determining the fate of hair cells exposed to stress.
Figures


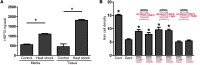

Comment in
-
The future of molecular chaperones and beyond.J Clin Invest. 2013 Aug;123(8):3206-8. doi: 10.1172/jci70799. J Clin Invest. 2013. PMID: 24063055 Free PMC article.
References
-
- Forge A, Schacht J. Aminoglycoside antibiotics. Audiol Neurootol. 2000;5(1):3–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

