Overcoming preexisting humoral immunity to AAV using capsid decoys
- PMID: 23863832
- PMCID: PMC4095828
- DOI: 10.1126/scitranslmed.3005795
Overcoming preexisting humoral immunity to AAV using capsid decoys
Abstract
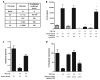
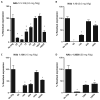
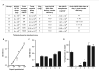
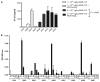
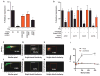
Adeno-associated virus (AAV) vectors delivered through the systemic circulation successfully transduce various target tissues in animal models. However, similar attempts in humans have been hampered by the high prevalence of neutralizing antibodies to AAV, which completely block vector transduction. We show in both mouse and nonhuman primate models that addition of empty capsid to the final vector formulation can, in a dose-dependent manner, adsorb these antibodies, even at high titers, thus overcoming their inhibitory effect. To further enhance the safety of the approach, we mutated the receptor binding site of AAV2 to generate an empty capsid mutant that can adsorb antibodies but cannot enter a target cell. Our work suggests that optimizing the ratio of full/empty capsids in the final formulation of vector, based on a patient's anti-AAV titers, will maximize the efficacy of gene transfer after systemic vector delivery.
Conflict of interest statement
Figures
References
-
- Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C, O'Beirne J, Smith K, Pasi J, Glader B, Rustagi P, Ng CY, Kay MA, Zhou J, Spence Y, Morton CL, Allay J, Coleman J, Sleep S, Cunningham JM, Srivastava D, Basner-Tschakarjan E, Mingozzi F, High KA, Gray JT, Reiss UM, Nienhuis AW, Davidoff AM. Adenovirus-associated virus vector–mediated gene transfer in hemophilia B. N Engl J Med. 2011;365:2357–2365. - PMC - PubMed
-
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, Dake M, Kaye R, Razavi M, Zajko A, Zehnder J, Rustagi PK, Nakai H, Chew A, Leonard D, Wright JF, Lessard RR, Sommer JM, Tigges M, Sabatino D, Luk A, Jiang H, Mingozzi F, Couto L, Ertl HC, High KA, Kay MA. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. - PubMed
-
- Li H, Haurigot V, Doyon Y, Li T, Wong SY, Bhagwat AS, Malani N, Anguela XM, Sharma R, Ivanciu L, Murphy SL, Finn JD, Khazi FR, Zhou S, Paschon DE, Rebar EJ, Bushman FD, Gregory PD, Holmes MC, High KA. In vivo genome editing restores haemostasis in a mouse model of haemophilia. Nature. 2011;475:217–221. - PMC - PubMed
-
- Arruda VR, Stedman HH, Haurigot V, Buchlis G, Baila S, Favaro P, Chen Y, Franck HG, Zhou S, Wright JF, Couto LB, Jiang H, Pierce GF, Bellinger DA, Mingozzi F, Nichols TC, High KA. Peripheral transvenular delivery of adeno-associated viral vectors to skeletal muscle as a novel therapy for hemophilia B. Blood. 2010;115:4678–4688. - PMC - PubMed
-
- Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF, Masurier C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: Implications for gene therapy using AAV vectors. Hum Gene Ther. 2010;21:704–712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

