Mouse extraembryonic arterial vessels harbor precursors capable of maturing into definitive HSCs
- PMID: 23863896
- PMCID: PMC3790504
- DOI: 10.1182/blood-2012-12-470971
Mouse extraembryonic arterial vessels harbor precursors capable of maturing into definitive HSCs
Abstract
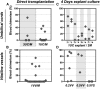
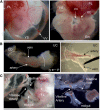
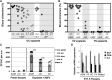
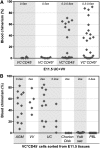
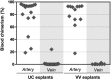
During mouse development, definitive hematopoietic stem cells (dHSCs) emerge by late E10.5 to E11 in several hematopoietic sites. Of them, the aorta-gonad-mesonephros (AGM) region drew particular attention owing to its capacity to autonomously initiate and expand dHSCs in culture, indicating its key role in HSC development. The dorsal aorta contains characteristic hematopoietic clusters and is the initial site of dHSC emergence, where they mature through vascular endothelial (VE)-cadherin(+)CD45(-)CD41(low) (type 1 pre-HSCs) and VE-cadherin(+)CD45(+) (type 2 pre-HSCs) intermediates. Although dHSCs were also found in other embryonic niches (placenta, yolk sac, and extraembryonic vessels), attempts to detect their HSC initiating potential have been unsuccessful to date. Extraembryonic arterial vessels contain hematopoietic clusters, suggesting that they develop HSCs, but functional evidence for this has been lacking. Here we show that umbilical cord and vitelline arteries (VAs), but not veins, contain pre-HSCs capable of maturing into dHSCs in the presence of exogenous interleukin 3, although in fewer numbers than the AGM region, and that pre-HSC activity in VAs increases with proximity to the embryo proper. Our functional data strongly suggest that extraembryonic arteries can actively contribute to adult hematopoiesis.
Figures


References
-
- Zeigler BM, Sugiyama D, Chen M, Guo Y, Downs KM, Speck NA. The allantois and chorion, when isolated before circulation or chorio-allantoic fusion, have hematopoietic potential. Development. 2006;133(21):4183–4192. - PubMed
-
- Corbel C, Salaün J, Belo-Diabangouaya P, Dieterlen-Lièvre F. Hematopoietic potential of the pre-fusion allantois. Dev Biol. 2007;301(2):478–488. - PubMed
-
- Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126(22):5073–5084. - PubMed
-
- Cumano A, Ferraz JC, Klaine M, Di Santo JP, Godin I. Intraembryonic, but not yolk sac hematopoietic precursors, isolated before circulation, provide long-term multilineage reconstitution. Immunity. 2001;15(3):477–485. - PubMed
-
- Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86(6):897–906. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

