Evolutionary biochemistry: revealing the historical and physical causes of protein properties
- PMID: 23864121
- PMCID: PMC4418793
- DOI: 10.1038/nrg3540
Evolutionary biochemistry: revealing the historical and physical causes of protein properties
Abstract
The repertoire of proteins and nucleic acids in the living world is determined by evolution; their properties are determined by the laws of physics and chemistry. Explanations of these two kinds of causality - the purviews of evolutionary biology and biochemistry, respectively - are typically pursued in isolation, but many fundamental questions fall squarely at the interface of fields. Here we articulate the paradigm of evolutionary biochemistry, which aims to dissect the physical mechanisms and evolutionary processes by which biological molecules diversified and to reveal how their physical architecture facilitates and constrains their evolution. We show how an integration of evolution with biochemistry moves us towards a more complete understanding of why biological molecules have the properties that they do.
Figures
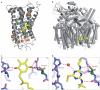

References
-
-
Anfinsen C. Molecular Basis of Evolution. John Wiley & Sons; 1959. This is a prescient early attempt by a Nobel-prize-winning biochemist to consider how chemistry might shape protein evolution.
-
-
- Florkin M. Biochemical Evolution. Academic Press; 1949.
-
- Zuckerkandl E, Pauling L. Molecules as documents of evolutionary history. J. Theor. Biol. 1965;8:357–366. - PubMed
-
-
Zuckerkandl E, Pauling L. Evolving Genes and Proteins. Bryson; 1965. Two chemists defend the potential contributions of biochemistry to evolutionary knowledge at a 1964 conference that brought molecular biologists and classical evolutionary biologists together.
-
-
- Pauling L, Zuckerkandl E. Chemical paleogenetics: molecular ‘restoration studies’ of extinct forms of life. Acta Chem. Scand. 1963;17:S9–S16.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

