Regulation of epileptiform discharges in rat neocortex by HCN channels
- PMID: 23864381
- PMCID: PMC3798942
- DOI: 10.1152/jn.00955.2012
Regulation of epileptiform discharges in rat neocortex by HCN channels
Abstract
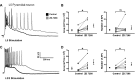
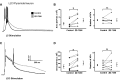
Hyperpolarization-activated, cyclic nucleotide-gated, nonspecific cation (HCN) channels have a well-characterized role in regulation of cellular excitability and network activity. The role of these channels in control of epileptiform discharges is less thoroughly understood. This is especially pertinent given the altered HCN channel expression in epilepsy. We hypothesized that inhibition of HCN channels would enhance bicuculline-induced epileptiform discharges. Whole cell recordings were obtained from layer (L)2/3 and L5 pyramidal neurons and L1 and L5 GABAergic interneurons. In the presence of bicuculline (10 μM), HCN channel inhibition with ZD 7288 (20 μM) significantly increased the magnitude (defined as area) of evoked epileptiform events in both L2/3 and L5 neurons. We recorded activity associated with epileptiform discharges in L1 and L5 interneurons to test the hypothesis that HCN channels regulate excitatory synaptic inputs differently in interneurons versus pyramidal neurons. HCN channel inhibition increased the magnitude of epileptiform events in both L1 and L5 interneurons. The increased magnitude of epileptiform events in both pyramidal cells and interneurons was due to an increase in network activity, since holding cells at depolarized potentials under voltage-clamp conditions to minimize HCN channel opening did not prevent enhancement in the presence of ZD 7288. In neurons recorded with ZD 7288-containing pipettes, bath application of the noninactivating inward cationic current (Ih) antagonist still produced increases in epileptiform responses. These results show that epileptiform discharges in disinhibited rat neocortex are modulated by HCN channels.
Keywords: HCN channels; Ih; epilepsy; epileptiform discharges; inhibitory interneurons; neocortex.
Figures



References
-
- Alefeld M, Sutor B, Luhmann HJ. Pattern and pharmacology of propagating epileptiform discharges in mouse cerebral cortex. Exp Neurol 153: 113–122, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

