Functions of the hydrophilic channels in protonmotive cytochrome c oxidase
- PMID: 23864498
- PMCID: PMC3730678
- DOI: 10.1098/rsif.2013.0183
Functions of the hydrophilic channels in protonmotive cytochrome c oxidase
Abstract
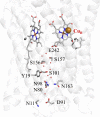
The structures and functions of hydrophilic channels in electron-transferring membrane proteins are discussed. A distinction is made between proton channels that can conduct protons and dielectric channels that are non-conducting but can dielectrically polarize in response to the introduction of charge changes in buried functional centres. Functions of the K, D and H channels found in A1-type cytochrome c oxidases are reviewed in relation to these ideas. Possible control of function by dielectric channels and their evolutionary relation to proton channels is explored.
Keywords: charge transfer; dielectric properties; electrostatics; hydrophilic channels; proton transfer; redox proteins.
Figures
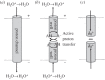
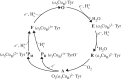
 and a neutral tyrosine radical). In the natural cycle, PM is reduced when a further electron is available from haem a, together with a charge-compensating substrate proton, to form F (ferryl haem a3,
and a neutral tyrosine radical). In the natural cycle, PM is reduced when a further electron is available from haem a, together with a charge-compensating substrate proton, to form F (ferryl haem a3,  , tyrosine ground state). If an electron is already available haem a when A is formed, A is instead transformed into the PR intermediate (ferryl haem a3,
, tyrosine ground state). If an electron is already available haem a when A is formed, A is instead transformed into the PR intermediate (ferryl haem a3,  , tyrosinate)—this is the case in flow-flash experiments described in the text in which fully reduced CcO (the FR state, with all four redox centres reduced) reacts with O2. PR is subsequently converted to F by uptake of a substrate proton. F is converted back to into the O state with a further electron from haem a together with a charge-compensating substrate proton. The P → F → O steps are often referred to as the ‘oxidative’ part of the reaction cycle. Each electron transfer into the BNC from haem a is generally (but see text) thought to be coupled to translocation of a proton across the membrane (not shown). See text for further details. Several quite different nomenclatures are now used in the primary CcO literature for these and additional intermediates; those above are most commonly used. However, these oxygen intermediates can be equated with those classically discussed in globins, P450s and peroxidases [48] as follows: A is equivalent to the oxyferrous state of globins and the equivalent ferric-superoxo ‘compound III’ of P450s and peroxidases; PM is equivalent to the ferryl-radical ‘compound I’; F and PR are equivalent to the ferryl ‘compound II’ with or without a charge-compensating proton, respectively.
, tyrosinate)—this is the case in flow-flash experiments described in the text in which fully reduced CcO (the FR state, with all four redox centres reduced) reacts with O2. PR is subsequently converted to F by uptake of a substrate proton. F is converted back to into the O state with a further electron from haem a together with a charge-compensating substrate proton. The P → F → O steps are often referred to as the ‘oxidative’ part of the reaction cycle. Each electron transfer into the BNC from haem a is generally (but see text) thought to be coupled to translocation of a proton across the membrane (not shown). See text for further details. Several quite different nomenclatures are now used in the primary CcO literature for these and additional intermediates; those above are most commonly used. However, these oxygen intermediates can be equated with those classically discussed in globins, P450s and peroxidases [48] as follows: A is equivalent to the oxyferrous state of globins and the equivalent ferric-superoxo ‘compound III’ of P450s and peroxidases; PM is equivalent to the ferryl-radical ‘compound I’; F and PR are equivalent to the ferryl ‘compound II’ with or without a charge-compensating proton, respectively.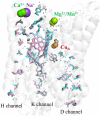

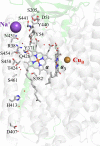
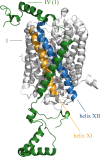
References
-
- Mitchell P. 1966. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Bodmin, UK: Glynn; Research Ltd - PubMed
-
- Mitchell P. 1968. Chemiosmotic coupling and energy transduction. Bodmin, UK: Glynn Research Ltd
-
- Junge W, Haumann M, Mulkidjanian A, Clausen J. 2002. Electrostatics and proton transfer in photosynthetic water oxidation. Phil. Trans. R. Soc. Lond. B 357, 1407–1418 (doi:10.1098/rstb.2002.1137) - DOI - PMC - PubMed
-
- Takahashi E, Wraight CA. 1992. Proton and electron transfer in the acceptor quinone complex of Rhodobacter sphaeroides reaction centers: characterization of site-directed mutants of the two ionizable residues, GluL212 and AspL213, in the QB binding site. Biochemistry 31, 855–867 (doi:10.1021/bi00118a031) - DOI - PubMed
-
- Mulkidjanian AY, Heberle J, Cherepanov DA. 2006. Protons at interfaces: implications for biological energy conversion. Biochim. Biophys. Acta Bioenerg. 1757, 913–930 (doi:10.1016/j.bbabio.2006.02.015) - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

