The effect of age and weight on vancomycin serum trough concentrations in pediatric patients
- PMID: 23864541
- PMCID: PMC3842376
- DOI: 10.1002/phar.1331
The effect of age and weight on vancomycin serum trough concentrations in pediatric patients
Abstract
Background: Vancomycin treatment failure has been associated with low serum vancomycin trough concentrations, prompting recommendations to increase the daily doses in adults and children. Despite more aggressive vancomycin dosing, there continues to be significant variability in vancomycin trough concentrations in pediatric patients.
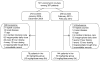
Methods: To determine if vancomycin trough concentrations in pediatric patients differ by age and weight, we reviewed records of hospitalized patients who received vancomycin between 2008 and 2012. Patients were divided into groups that received vancomycin 40 mg/kg/day (2008-2009) or 60 mg/kg/day (2010-2012). Vancomycin trough concentrations were compared between groups and within the 60 mg/kg/day group, stratified by patient age and weight.
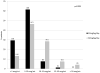
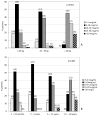
Results: After increasing the vancomycin dose from 40 to 60 mg/kg/day, initial trough concentrations increased significantly in patients younger than 2 and greater than 6 years of age, but not in patients between the ages of 2 and 5 years. In the 60 mg/kg/day group, only 16.7% of patients between 2 and 5 years of age had initial trough concentrations in the therapeutic range (10-20 μg/ml). Initial trough concentrations were therapeutic in a greater proportion of patients ages 6-12 years (38.7%) and 13-18 years (63.0%). Patients between the ages of 13 and 18 had the highest proportion of supratherapeutic initial vancomycin trough concentrations (14.8%). Patients weighing over 50 kg had significantly higher trough concentrations than patients 50 kg or less (17.1 μg/ml vs 9.3 μg/ml, p<0.001).
Conclusion: Although increasing the vancomycin dose from 40 to 60 mg/kg/day led to a significant increase in vancomycin trough concentrations, a large proportion of patients receiving 60 mg/kg/day of vancomycin had trough concentrations outside of the therapeutic range. Specifically, patients younger than 6 years tended to have low trough concentrations, whereas adolescents and children over 50 kg were more likely to have elevated trough concentrations. Vancomycin dosing strategies in pediatric patients should consider age and weight as well as renal function and indication.
Keywords: age; pediatric; vancomycin; weight.
© 2013 Pharmacotherapy Publications, Inc.
Figures
References
-
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18–55. - PubMed
-
- Kullar R, Davis SL, Levine DP, Rybak MJ. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis. 2011;52:975–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

