SLC4A11 is an EIPA-sensitive Na(+) permeable pHi regulator
- PMID: 23864606
- PMCID: PMC3798668
- DOI: 10.1152/ajpcell.00056.2013
SLC4A11 is an EIPA-sensitive Na(+) permeable pHi regulator
Abstract
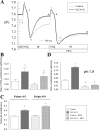
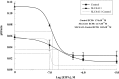
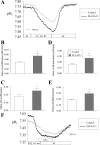
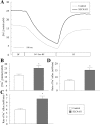
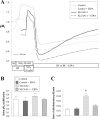
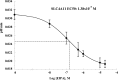
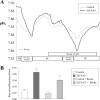
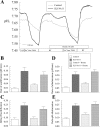
Slc4a11, a member of the solute linked cotransporter 4 family that is comprised predominantly of bicarbonate transporters, was described as an electrogenic 2Na(+)-B(OH)4(-) (borate) cotransporter and a Na(+)-2OH(-) cotransporter. The goal of the current study was to confirm and/or clarify the function of SLC4A11. In HEK293 cells transfected with SLC4A11 we tested if SLC4A11 is a: 1) Na(+)-HCO3(-) cotransporter, 2) Na(+)-OH(-)(H(+)) transporter, and/or 3) Na(+)-B(OH)4(-) cotransporter. CO2/HCO3(-) perfusion yielded no significant differences in rate or extent of pHi changes or Na(+) flux in SLC4A11-transfected compared with control cells. Similarly, in CO2/HCO3(-), acidification on removal of Na(+) and alkalinization on Na(+) add back were not significantly different between control and transfected indicating that SLC4A11 does not have Na(+)-HCO3(-) cotransport activity. In the absence of CO2/HCO3(-), SLC4A11-transfected cells showed higher resting intracelllular Na(+) concentration ([Na(+)]i; 25 vs. 17 mM), increased NH4(+)-induced acidification and increased acid recovery rate (160%) after an NH4 pulse. Na(+) efflux and influx were faster (80%) following Na(+) removal and add back, respectively, indicative of Na(+)-OH(-)(H(+)) transport by SLC4A11. The increased alkalinization recovery was confirmed in NHE-deficient PS120 cells demonstrating that SLC4A11 is a bonafide Na(+)-OH(-)(H(+)) transporter and not an activator of NHEs. SLC4A11-mediated H(+) efflux is inhibited by 5-(N-ethyl-N-isopropyl) amiloride (EIPA; EC50: 0.1 μM). The presence of 10 mM borate did not alter dpHi/dt or ΔpH during a Na(+)-free pulse in SLC4A11-transfected cells. In summary our results show that SLC4A11 is not a bicarbonate or borate-linked transporter but has significant EIPA-sensitive Na(+)-OH(-)(H(+)) and NH4(+) permeability.
Keywords: Na+ permeability; SLC4A11; bicarbonate; borate; pH.
Figures
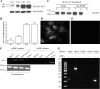
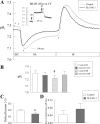
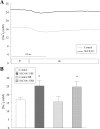
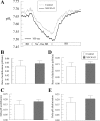

References
-
- Amlal H, Wang Z, Burnham C, Soleimani M. Functional characterization of a cloned human kidney Na+:HCO3− cotransporter. J Biol Chem 273: 16810–16815, 1998 - PubMed
-
- Bonanno JA, Giasson C. Intracellular pH regulation in fresh and cultured bovine corneal endothelium. I. Na+/H+ exchange in the absence and presence of HCO3−. Invest Ophthalmol Vis Sci 33: 3058–3067, 1992 - PubMed
-
- Bonanno JA, Giasson C. Intracellular pH regulation in fresh and cultured bovine corneal endothelium. II. Na+:HCO3− cotransport and Cl−/HCO3− exchange. Invest Ophthalmol Vis Sci 33: 3068–3079, 1992 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

