Plasma membrane domains enriched in cortical endoplasmic reticulum function as membrane protein trafficking hubs
- PMID: 23864710
- PMCID: PMC3756922
- DOI: 10.1091/mbc.E12-12-0895
Plasma membrane domains enriched in cortical endoplasmic reticulum function as membrane protein trafficking hubs
Abstract
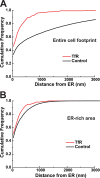
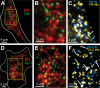
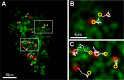
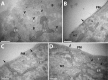
In mammalian cells, the cortical endoplasmic reticulum (cER) is a network of tubules and cisterns that lie in close apposition to the plasma membrane (PM). We provide evidence that PM domains enriched in underlying cER function as trafficking hubs for insertion and removal of PM proteins in HEK 293 cells. By simultaneously visualizing cER and various transmembrane protein cargoes with total internal reflectance fluorescence microscopy, we demonstrate that the majority of exocytotic delivery events for a recycled membrane protein or for a membrane protein being delivered to the PM for the first time occur at regions enriched in cER. Likewise, we observed recurring clathrin clusters and functional endocytosis of PM proteins preferentially at the cER-enriched regions. Thus the cER network serves to organize the molecular machinery for both insertion and removal of cell surface proteins, highlighting a novel role for these unique cellular microdomains in membrane trafficking.
Figures




References
-
- Antonucci DE, Lim ST, Vassanelli S, Trimmer JS. Dynamic localization and clustering of dendritic Kv2.1 voltage-dependent potassium channels in developing hippocampal neurons. Neuroscience. 2001;108:69–81. - PubMed
-
- Barlow AL, Macleod A, Noppen S, Sanderson J, Guerin CJ. Colocalization analysis in fluorescence micrographs: verification of a more accurate calculation of Pearson's correlation coefficient. Microsc Microanal. 2010;16:710–724. - PubMed
-
- Bellve KD, Leonard D, Standley C, Lifshitz LM, Tuft RA, Hayakawa A, Corvera S, Fogarty KE. Plasma membrane domains specialized for clathrin-mediated endocytosis in primary cells. J Biol Chem. 2006;281:16139–16146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

