Liquid-liquid equilibrium of poly(ethylene glycol) 6000 + sodium succinate + water system at different temperatures
- PMID: 23864835
- PMCID: PMC3706021
- DOI: 10.1155/2013/819259
Liquid-liquid equilibrium of poly(ethylene glycol) 6000 + sodium succinate + water system at different temperatures
Abstract
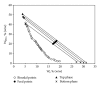
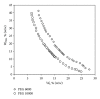
Phase diagrams and the compositions of coexisting phases of poly(ethylene glycol) (PEG) 6000 + sodium succinate + water system have been determined experimentally at 298.15, 308.15, and 318.15 K. The effects of temperature on the binodal curve and tie lines have been studied. The binodal curves were successfully fitted to a nonlinear equation relating the concentrations of PEG 6000 and sodium succinate, and the coefficients were estimated for the formentioned systems (low AARD, high R(2), and low SD). Tie-line compositions were estimated and correlated using Othmer-Tobias and Bancroft equations, and the parameters were reported. The effect of temperature on the phase-forming ability has been studied by fitting the binodal data to a Setschenow-type equation for each temperature. The effective excluded volume (EEV) values were also calculated from the binodal data, and it was found out that the values increased with an increase in the temperature. Furthermore, the effect of MW of PEG on the phase diagram has been studied and verified.
Figures



References
-
- Albertsson PA. Partitioning of Cell Particles and Macromolecules. 3rd edition. New York, NY, USA: John Wiley & Sons; 1987.
-
- Naganagouda K, Mulimani VH. Aqueous two-phase extraction (ATPE): an attractive and economically viable technology for downstream processing of Aspergillus oryzaeα-galactosidase. Process Biochemistry. 2008;43(11):1293–1299.
-
- Trindade IP, Diogo MM, Prazeres DMF, Marcos JC. Purification of plasmid DNA vectors by aqueous two-phase extraction and hydrophobic interaction chromatography. Journal of Chromatography A. 2005;1082(2):176–184. - PubMed
-
- Aguilar O, Albiter V, Serrano-Carreón L, Rito-Palomares M. Direct comparison between ion-exchange chromatography and aqueous two-phase processes for the partial purification of penicillin acylase produced by E. coli . Journal of Chromatography B. 2006;835(1-2):77–83. - PubMed
-
- Ivetic DZ, Sciban MB, Vasic VM, Kukic DV, Prodanovic JM, Antov MG. Evaluation of possibility of textile dye removal from wastewater by aqueous two-phase extraction. Desalination and Water Treatment. 2012;51:1–6.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

