Multipotent mesenchymal stem cells from human subacromial bursa: potential for cell based tendon tissue engineering
- PMID: 23865619
- PMCID: PMC3875151
- DOI: 10.1089/ten.TEA.2013.0197
Multipotent mesenchymal stem cells from human subacromial bursa: potential for cell based tendon tissue engineering
Abstract
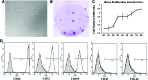
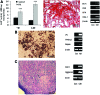
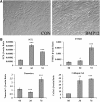
Rotator cuff injuries are a common clinical problem either as a result of overuse or aging. Biological approaches to tendon repair that involve use of scaffolding materials or cell-based approaches are currently being investigated. The cell-based approaches are focused on applying multipotent mesenchymal stem cells (MSCs) mostly harvested from bone marrow. In the present study, we focused on characterizing cells harvested from tissues associated with rotator cuff tendons based on an assumption that these cells would be more appropriate for tendon repair. We isolated MSCs from bursa tissue associated with rotator cuff tendons and characterized them for multilineage differentiation in vitro and in vivo. Human bursa was obtained from patients undergoing rotator cuff surgery and cells within were isolated using collagenase and dispase digestion. The cells isolated from the tissues were characterized for osteoblastic, adipogenic, chondrogenic, and tenogenic differentiation in vitro and in vivo. The results showed that the cells isolated from bursa tissue exhibited MSCs characteristics as evidenced by the expression of putative cell surface markers attributed to MSCs. The cells exhibited high proliferative capacity and differentiated toward cells of mesenchymal lineages with high efficiency. Bursa-derived cells expressed markers of tenocytes when treated with bone morphogenetic protein-12 (BMP-12) and assumed aligned morphology in culture. Bursa cells pretreated with BMP-12 and seeded in ceramic scaffolds formed extensive bone, as well as tendon-like tissue in vivo. Bone formation was demonstrated by histological analysis and immunofluorescence for DMP-1 in tissue sections made from the scaffolds seeded with the cells. Tendon-like tissue formed in vivo consisted of parallel collagen fibres typical of tendon tissues. Bursa-derived cells also formed a fibrocartilagenous tissue in the ceramic scaffolds. Taken together, the results demonstrate a new source of MSCs with a high potential for application in tendon repair.
Figures



Similar articles
-
Isolation and characterization of human mesenchymal stem cells derived from shoulder tissues involved in rotator cuff tears.Am J Sports Med. 2013 Mar;41(3):657-68. doi: 10.1177/0363546512473269. Epub 2013 Jan 31. Am J Sports Med. 2013. PMID: 23371475
-
Tissue-engineered collagen matrix loaded with rat adipose-derived stem cells/human amniotic mesenchymal stem cells for rotator cuff tendon-bone repair.Int J Biol Macromol. 2024 Dec;282(Pt 4):137144. doi: 10.1016/j.ijbiomac.2024.137144. Epub 2024 Oct 31. Int J Biol Macromol. 2024. PMID: 39488324
-
The application of BMP-12-overexpressing mesenchymal stem cells loaded 3D-printed PLGA scaffolds in rabbit rotator cuff repair.Int J Biol Macromol. 2019 Oct 1;138:79-88. doi: 10.1016/j.ijbiomac.2019.07.041. Epub 2019 Jul 8. Int J Biol Macromol. 2019. PMID: 31295489
-
Tendon tissue engineering: Current progress towards an optimized tenogenic differentiation protocol for human stem cells.Acta Biomater. 2022 Jun;145:25-42. doi: 10.1016/j.actbio.2022.04.028. Epub 2022 Apr 22. Acta Biomater. 2022. PMID: 35470075 Review.
-
Regenerative medicine in rotator cuff injuries.Biomed Res Int. 2014;2014:129515. doi: 10.1155/2014/129515. Epub 2014 Aug 13. Biomed Res Int. 2014. PMID: 25184132 Free PMC article. Review.
Cited by
-
Decreased Colony-Forming Ability of Subacromial Bursa-Derived Cells During Revision Arthroscopic Rotator Cuff Repair.Arthrosc Sports Med Rehabil. 2021 May 14;3(4):e1047-e1054. doi: 10.1016/j.asmr.2021.03.010. eCollection 2021 Aug. Arthrosc Sports Med Rehabil. 2021. PMID: 34430884 Free PMC article.
-
Advances in biology and mechanics of rotator cuff repair.Knee Surg Sports Traumatol Arthrosc. 2015 Feb;23(2):530-41. doi: 10.1007/s00167-014-3487-2. Epub 2015 Jan 9. Knee Surg Sports Traumatol Arthrosc. 2015. PMID: 25573661 Review.
-
Use of stem cells and growth factors in rotator cuff tendon repair.Eur J Orthop Surg Traumatol. 2019 May;29(4):747-757. doi: 10.1007/s00590-019-02366-x. Epub 2019 Jan 9. Eur J Orthop Surg Traumatol. 2019. PMID: 30627922 Review.
-
Advances in biologic augmentation for rotator cuff repair.Ann N Y Acad Sci. 2016 Nov;1383(1):97-114. doi: 10.1111/nyas.13267. Epub 2016 Oct 17. Ann N Y Acad Sci. 2016. PMID: 27750374 Free PMC article. Review.
-
Biological analysis of flexor tendon repair-failure stump tissue: A potential recycling of tissue for tendon regeneration.Bone Joint Res. 2019 Jul 5;8(6):232-245. doi: 10.1302/2046-3758.86.BJR-2018-0239.R1. eCollection 2019 Jun. Bone Joint Res. 2019. PMID: 31346451 Free PMC article.
References
-
- Pennnisi E.Tending tender tendons. Science 295,1011, 2002 - PubMed
-
- Goh J., Ouyang H.W., Teoh S.H., Chan C.K., and Lee E.H.Tissue-engineering approach to the repair and regeneration of tendons and ligaments. Tissue Eng 9,S31, 2003 - PubMed
-
- Vunjak-Novakovic G., Altman G., Horan R., and Kaplan D.L.Tissue engineering of ligaments. Annu Rev Biomed Eng 6,131, 2004 - PubMed
-
- Coons D.A., and Alan Barber F.Tendon graft substitutes-rotator cuff patches. Sports Med Arthrosc 14,185, 2006 - PubMed
-
- Sclamberg S.G., Tibone J.E., Itamura J.M., and Kasraeian S.Six-month magnetic resonance imaging follow-up of large and massive rotator cuff repairs reinforced with porcine small intestinal submucosa. J Shoulder Elbow Surg 13,538, 2004 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

