Competition between homodimerization and cholesterol binding to the C99 domain of the amyloid precursor protein
- PMID: 23865807
- PMCID: PMC3758481
- DOI: 10.1021/bi400735x
Competition between homodimerization and cholesterol binding to the C99 domain of the amyloid precursor protein
Abstract
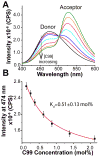
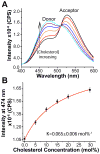
The 99-residue transmembrane C-terminal domain (C99, also known as β-CTF) of the amyloid precursor protein (APP) is the product of the β-secretase cleavage of the full-length APP and is the substrate for γ-secretase cleavage. The latter cleavage releases the amyloid-β polypeptides that are closely associated with Alzheimer's disease. C99 is thought to form homodimers; however, the free energy in favor of dimerization has not previously been quantitated. It was also recently documented that cholesterol forms a 1:1 complex with monomeric C99 in bicelles. Here, the affinities for both homodimerization and cholesterol binding to C99 were measured in bilayered lipid vesicles using both electron paramagnetic resonance (EPR) and Förster resonance energy transfer (FRET) methods. Homodimerization and cholesterol binding were seen to be competitive processes that center on the transmembrane G₇₀₀XXXG₇₀₄XXXG₇₀₈ glycine-zipper motif and adjacent Gly709. On one hand, the observed Kd for cholesterol binding (Kd = 2.7 ± 0.3 mol %) is on the low end of the physiological cholesterol concentration range in mammalian cell membranes. On the other hand, the observed K(d) for homodimerization (K(d) = 0.47 ± 0.15 mol %) likely exceeds the physiological concentration range for C99. These results suggest that the 1:1 cholesterol/C99 complex will be more highly populated than C99 homodimers under most physiological conditions. These observations are of relevance for understanding the γ-secretase cleavage of C99.
Figures





Similar articles
-
Dimerization of the transmembrane domain of amyloid precursor protein is determined by residues around the γ-secretase cleavage sites.J Biol Chem. 2017 Sep 22;292(38):15826-15837. doi: 10.1074/jbc.M117.789669. Epub 2017 Aug 8. J Biol Chem. 2017. PMID: 28790170 Free PMC article.
-
The amyloid precursor protein has a flexible transmembrane domain and binds cholesterol.Science. 2012 Jun 1;336(6085):1168-71. doi: 10.1126/science.1219988. Science. 2012. PMID: 22654059 Free PMC article.
-
Structural studies of the transmembrane C-terminal domain of the amyloid precursor protein (APP): does APP function as a cholesterol sensor?Biochemistry. 2008 Sep 9;47(36):9428-46. doi: 10.1021/bi800993c. Epub 2008 Aug 15. Biochemistry. 2008. PMID: 18702528 Free PMC article.
-
Cholesterol as a co-solvent and a ligand for membrane proteins.Protein Sci. 2014 Jan;23(1):1-22. doi: 10.1002/pro.2385. Epub 2013 Nov 18. Protein Sci. 2014. PMID: 24155031 Free PMC article. Review.
-
MAM and C99, key players in the pathogenesis of Alzheimer's disease.Int Rev Neurobiol. 2020;154:235-278. doi: 10.1016/bs.irn.2020.03.016. Epub 2020 Jul 22. Int Rev Neurobiol. 2020. PMID: 32739006 Review.
Cited by
-
Lipid Binding Controls Dimerization of the Coat Protein p24 Transmembrane Helix.Biophys J. 2019 Nov 5;117(9):1554-1562. doi: 10.1016/j.bpj.2019.09.021. Epub 2019 Sep 23. Biophys J. 2019. PMID: 31627840 Free PMC article.
-
Comparing the structural dynamics of the human KCNE3 in reconstituted micelle and lipid bilayered vesicle environments.Biochim Biophys Acta Biomembr. 2022 Oct 1;1864(10):183974. doi: 10.1016/j.bbamem.2022.183974. Epub 2022 Jun 15. Biochim Biophys Acta Biomembr. 2022. PMID: 35716725 Free PMC article.
-
Both positional and chemical variables control in vitro proteolytic cleavage of a presenilin ortholog.J Biol Chem. 2018 Mar 30;293(13):4653-4663. doi: 10.1074/jbc.RA117.001436. Epub 2018 Jan 30. J Biol Chem. 2018. PMID: 29382721 Free PMC article.
-
The vexing complexity of the amyloidogenic pathway.Protein Sci. 2019 Jul;28(7):1177-1193. doi: 10.1002/pro.3606. Epub 2019 Apr 11. Protein Sci. 2019. PMID: 30897251 Free PMC article. Review.
-
Probing the interaction of the potassium channel modulating KCNE1 in lipid bilayers via solid-state NMR spectroscopy.Magn Reson Chem. 2017 Aug;55(8):754-758. doi: 10.1002/mrc.4589. Epub 2017 Mar 16. Magn Reson Chem. 2017. PMID: 28233402 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

