Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia
- PMID: 23866823
- PMCID: PMC4172321
- DOI: 10.1016/j.jaci.2013.05.020
Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia
Erratum in
- J Allergy Clin Immunol. 2014 Apr;133(4):1232
Abstract
Background: Many asthmatic patients exhibit sputum eosinophilia associated with exacerbations. Benralizumab targets eosinophils by binding IL-5 receptor α, inducing apoptosis through antibody-dependent cell-mediated cytotoxicity.
Objectives: We sought to evaluate the safety of benralizumab in adults with eosinophilic asthma and its effects on eosinophil counts in airway mucosal/submucosal biopsy specimens, sputum, bone marrow, and peripheral blood.
Methods: In this multicenter, double-blind, placebo-controlled phase I study, 13 subjects were randomized to single-dose intravenous placebo or 1 mg/kg benralizumab (day 0; cohort 1), and 14 subjects were randomized to 3 monthly subcutaneous doses of placebo or 100 or 200 mg of benralizumab (days 0, 28, and 56; cohort 2). Cohorts 1 and 2 were consecutive.
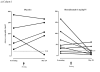
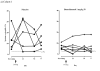
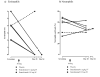
Results: The incidence of adverse events was similar between groups. No serious adverse events related to benralizumab occurred. In cohort 1 intravenous benralizumab produced a median decrease from baseline of 61.9% in airway mucosal eosinophil counts (day 28; placebo: +19.6%; P = .28), as well as an 18.7% decrease (day 21) in sputum and a 100% decrease (day 28) in blood counts. Eosinophils were not detectable in bone marrow of benralizumab-treated subjects (day 28, n = 4). In cohort 2 subcutaneous benralizumab demonstrated a combined (100 + 200 mg) median reduction of 95.8% in airway eosinophil counts (day 84; placebo, 46.7%; P = .06), as well as an 89.9% decrease (day 28) in sputum and a 100% decrease (day 84) in blood counts.
Conclusion: Single-dose intravenous and multiple-dose subcutaneous benralizumab reduced eosinophil counts in airway mucosa/submucosa and sputum and suppressed eosinophil counts in bone marrow and peripheral blood. The safety profile supports further development. Additional studies are needed to assess the clinical benefit in asthmatic patients.
Keywords: ADCC; AE; ATP; According to protocol; Adverse event; Antibody-dependent cell-mediated cytotoxicity; C-reactive protein; CPK; CRP; Creatine phosphokinase; Eosinophils; H&E; Hematoxylin and eosin; ICS; IL-5; IL-5 receptor; IL-5 receptors; IL-5R; IQR; Inhaled corticosteroid; Interquartile range; SABA; Short-acting β(2)-agonist; antibody-dependent cell-mediated cytotoxicity; asthma.
Copyright © 2013 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Figures







References
-
- Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360:1715–221. - PubMed
-
- Jayaram L, Pizzichini MM, Cook RJ, Boulet LP, Lemiere C, Pizzichini E, et al. Determining asthma treatment by monitoring sputum cell counts: effect on exacerbations. Eur Respir J. 2006;27:483–94. - PubMed
-
- Chlumský J, Striz I, Terl M, Vondracek J. Strategy aimed at reduction of sputum eosinophils decreases exacerbation rate in patients with asthma. J Int Med Res. 2006;34:129–39. - PubMed
-
- Silkoff PE, Lent AM, Busacker AA, Katial RK, Balzar S, Strand M, et al. Exhaled nitric oxide identifies the persistent eosinophilic phenotype in severe refractory asthma. J Allergy Clin Immunol. 2005;116:1249–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

