Posttranslational modification and conformational state of heat shock protein 90 differentially affect binding of chemically diverse small molecule inhibitors
- PMID: 23867252
- PMCID: PMC3759666
- DOI: 10.18632/oncotarget.1099
Posttranslational modification and conformational state of heat shock protein 90 differentially affect binding of chemically diverse small molecule inhibitors
Abstract
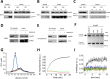
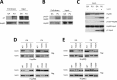
Heat shock protein 90 (Hsp90) is an essential molecular chaperone in eukaryotes that facilitates the conformational maturation and function of a diverse protein clientele, including aberrant and/or over-expressed proteins that are involved in cancer growth and survival. A role for Hsp90 in supporting the protein homeostasis of cancer cells has buoyed interest in the utility of Hsp90 inhibitors as anti-cancer drugs. Despite the fact that all clinically evaluated Hsp90 inhibitors target an identical nucleotide-binding pocket in the N domain of the chaperone, the precise determinants that affect drug binding in the cellular environment remain unclear, and it is possible that chemically distinct inhibitors may not share similar binding preferences. Here we demonstrate that two chemically unrelated Hsp90 inhibitors, the benzoquinone ansamycin geldanamycin and the purine analog PU-H71, select for overlapping but not identical subpopulations of total cellular Hsp90, even though both inhibitors bind to an amino terminal nucleotide pocket and prevent N domain dimerization. Our data also suggest that PU-H71 is able to access a broader range of N domain undimerized Hsp90 conformations than is geldanamycin and is less affected by Hsp90 phosphorylation, consistent with its broader and more potent anti-tumor activity. A more complete understanding of the impact of the cellular milieu on small molecule inhibitor binding to Hsp90 should facilitate their more effective use in the clinic.
Figures


References
-
- Neckers L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol Med. 2002;8(4 Suppl):S55–61. - PubMed
-
- Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11(7):515–528. - PubMed
-
- Modi S, Stopeck A, Linden H, Solit D, Chandarlapaty S, Rosen N, D'Andrea G, Dickler M, Moynahan ME, Sugarman S, Ma W, Patil S, Norton L, Hannah AL, Hudis C. HSP90 inhibition is effective in breast cancer: a phase II trial of tanespimycin (17-AAG) plus trastuzumab in patients with HER2-positive metastatic breast cancer progressing on trastuzumab. Clin Cancer Res. 2011;17(15):5132–5139. - PubMed
-
- Modi S, Stopeck AT, Gordon MS, Mendelson D, Solit DB, Bagatell R, Ma W, Wheler J, Rosen N, Norton L, Cropp GF, Johnson RG, Hannah AL, Hudis CA. Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is safe and active in trastuzumab-refractory HER-2 overexpressing breast cancer: a phase I dose-escalation study. J Clin Oncol. 2007;25(34):5410–5417. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

