Macrophages regulate corpus luteum development during embryo implantation in mice
- PMID: 23867505
- PMCID: PMC3726148
- DOI: 10.1172/JCI60561
Macrophages regulate corpus luteum development during embryo implantation in mice
Abstract
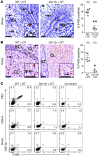
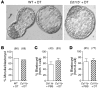
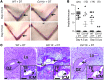
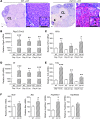
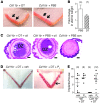
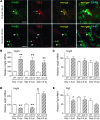
Macrophages are prominent in the uterus and ovary at conception. Here we utilize the Cd11b-Dtr mouse model of acute macrophage depletion to define the essential role of macrophages in early pregnancy. Macrophage depletion after conception caused embryo implantation arrest associated with diminished plasma progesterone and poor uterine receptivity. Implantation failure was alleviated by administration of bone marrow-derived CD11b+F4/80+ monocytes/macrophages. In the ovaries of macrophage-depleted mice, corpora lutea were profoundly abnormal, with elevated Ptgs2, Hif1a, and other inflammation and apoptosis genes and with diminished expression of steroidogenesis genes Star, Cyp11a1, and Hsd3b1. Infertility was rescued by exogenous progesterone, which confirmed that uterine refractoriness was fully attributable to the underlying luteal defect. In normally developing corpora lutea, macrophages were intimately juxtaposed with endothelial cells and expressed the proangiogenic marker TIE2. After macrophage depletion, substantial disruption of the luteal microvascular network occurred and was associated with altered ovarian expression of genes that encode vascular endothelial growth factors. These data indicate a critical role for macrophages in supporting the extensive vascular network required for corpus luteum integrity and production of progesterone essential for establishing pregnancy. Our findings raise the prospect that disruption of macrophage-endothelial cell interactions underpinning corpus luteum development contributes to infertility in women in whom luteal insufficiency is implicated.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

