Tracking axonal action potential propagation on a high-density microelectrode array across hundreds of sites
- PMID: 23867868
- PMCID: PMC5419423
- DOI: 10.1038/ncomms3181
Tracking axonal action potential propagation on a high-density microelectrode array across hundreds of sites
Abstract
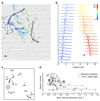
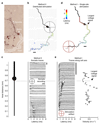
Axons are traditionally considered stable transmission cables, but evidence of the regulation of action potential propagation demonstrates that axons may have more important roles. However, their small diameters render intracellular recordings challenging, and low-magnitude extracellular signals are difficult to detect and assign. Better experimental access to axonal function would help to advance this field. Here we report methods to electrically visualize action potential propagation and network topology in cortical neurons grown over custom arrays, which contain 11,011 microelectrodes and are fabricated using complementary metal oxide semiconductor technology. Any neuron lying on the array can be recorded at high spatio-temporal resolution, and simultaneously precisely stimulated with little artifact. We find substantial velocity differences occurring locally within single axons, suggesting that the temporal control of a neuron's output may contribute to neuronal information processing.
Conflict of interest statement
Figures






References
-
- Shu Y, Hasenstaub A, Duque A, Yu Y, McCormick DA. Modulation of intracortical synaptic potentials by presynaptic somatic membrane potential. Nature. 2006;441:761–765. - PubMed
-
- Alle H, Geiger JR. Combined analog and action potential coding in hippocampal mossy fibers. Science. 2006;311:1290–1293. - PubMed
-
- Sasaki T, Matsuki N, Ikegaya Y. Action-potential modulation during axonal conduction. Science. 2011;331:599–601. - PubMed
-
- Izhikevich EM. Polychronization: computation with spikes. Neural Comput. 2006;18:245–282. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

