Reolysin is a novel reovirus-based agent that induces endoplasmic reticular stress-mediated apoptosis in pancreatic cancer
- PMID: 23868061
- PMCID: PMC3730429
- DOI: 10.1038/cddis.2013.259
Reolysin is a novel reovirus-based agent that induces endoplasmic reticular stress-mediated apoptosis in pancreatic cancer
Abstract
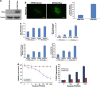
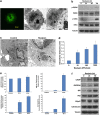
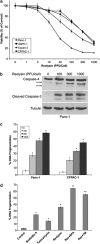
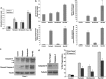
Activating mutation of KRas is a genetic alteration that occurs in the majority of pancreatic tumors and is therefore an ideal therapeutic target. The ability of reoviruses to preferentially replicate and induce cell death in transformed cells that express activated Ras prompted the development of a reovirus-based formulation for cancer therapy called Reolysin. We hypothesized that Reolysin exposure would trigger heavy production of viral products leading to endoplasmic reticular (ER) stress-mediated apoptosis. Here, we report that Reolysin treatment stimulated selective reovirus replication and decreased cell viability in KRas-transformed immortalized human pancreatic duct epithelial cells and pancreatic cancer cell lines. These effects were associated with increased expression of ER stress-related genes, ER swelling, cleavage of caspase-4, and splicing of XBP-1. Treatment with ER stress stimuli including tunicamycin, brefeldin A, and bortezomib (BZ) augmented the anticancer activity of Reolysin. Cotreatment with BZ and Reolysin induced the simultaneous accumulation of ubiquitinated and viral proteins, resulting in enhanced levels of ER stress and apoptosis in both in vitro and in vivo models of pancreatic cancer. Our collective results demonstrate that the abnormal protein accumulation induced by the combination of Reolysin and BZ promotes heightened ER stress and apoptosis in pancreatic cancer cells and provides the rationale for a phase I clinical trial further investigating the safety and efficacy of this novel strategy.
Figures

References
-
- Bos JL. Ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. - PubMed
-
- Fearon ER. K-ras gene mutation as a pathogenetic and diagnostic marker in human cancer. J Natl Cancer Inst. 1993;85:1978–1980. - PubMed
-
- Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3:459–465. - PubMed
-
- Kelly K, Nawrocki S, Mita A, Coffey M, Giles FJ, Mita M. Reovirus-based therapy for cancer. Expert Opin Biol Ther. 2009;9:817–830. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

