LRG1 promotes angiogenesis by modulating endothelial TGF-β signalling
- PMID: 23868260
- PMCID: PMC3836402
- DOI: 10.1038/nature12345
LRG1 promotes angiogenesis by modulating endothelial TGF-β signalling
Abstract
Aberrant neovascularization contributes to diseases such as cancer, blindness and atherosclerosis, and is the consequence of inappropriate angiogenic signalling. Although many regulators of pathogenic angiogenesis have been identified, our understanding of this process is incomplete. Here we explore the transcriptome of retinal microvessels isolated from mouse models of retinal disease that exhibit vascular pathology, and uncover an upregulated gene, leucine-rich alpha-2-glycoprotein 1 (Lrg1), of previously unknown function. We show that in the presence of transforming growth factor-β1 (TGF-β1), LRG1 is mitogenic to endothelial cells and promotes angiogenesis. Mice lacking Lrg1 develop a mild retinal vascular phenotype but exhibit a significant reduction in pathological ocular angiogenesis. LRG1 binds directly to the TGF-β accessory receptor endoglin, which, in the presence of TGF-β1, results in promotion of the pro-angiogenic Smad1/5/8 signalling pathway. LRG1 antibody blockade inhibits this switch and attenuates angiogenesis. These studies reveal a new regulator of angiogenesis that mediates its effect by modulating TGF-β signalling.
Figures
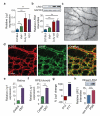
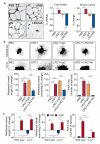
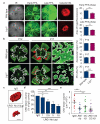


References
-
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. - PubMed
-
- Carmeliet P, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. - PubMed
-
- Ferrara N, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. - PubMed
-
- Holderfield MT, Hughes CC. Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-beta in vascular morphogenesis. Circ Res. 2008;102:637–652. - PubMed
-
- Chung AS, Ferrara N. Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol. 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

