Chemical reporters for biological discovery
- PMID: 23868317
- PMCID: PMC3866016
- DOI: 10.1038/nchembio.1296
Chemical reporters for biological discovery
Erratum in
- Nat Chem Biol. 2014 Mar;10(3):239
Abstract
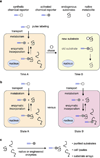
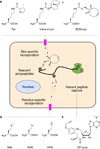
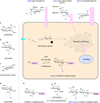
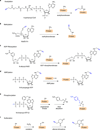
Functional tools are needed to understand complex biological systems. Here we review how chemical reporters in conjunction with bioorthogonal labeling methods can be used to image and retrieve nucleic acids, proteins, glycans, lipids and other metabolites in vitro, in cells as well as in whole organisms. By tagging these biomolecules, researchers can now monitor their dynamics in living systems and discover specific substrates of cellular pathways. These advances in chemical biology are thus providing important tools to characterize biological pathways and are poised to facilitate our understanding of human diseases.
Figures



References
-
- Prescher JA, Bertozzi CR. Chemistry in living systems. Nat Chem Biol. 2005;1:13–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

