Nanosensor dosimetry of mouse blood proteins after exposure to ionizing radiation
- PMID: 23868657
- PMCID: PMC3715761
- DOI: 10.1038/srep02234
Nanosensor dosimetry of mouse blood proteins after exposure to ionizing radiation
Abstract
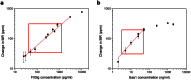
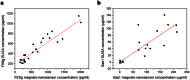
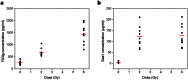
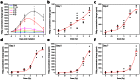
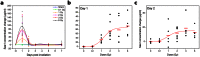
Giant magnetoresistive (GMR) nanosensors provide a novel approach for measuring protein concentrations in blood for medical diagnosis. Using an in vivo mouse radiation model, we developed protocols for measuring Flt3 ligand (Flt3lg) and serum amyloid A1 (Saa1) in small amounts of blood collected during the first week after X-ray exposures of sham, 0.1, 1, 2, 3, or 6 Gy. Flt3lg concentrations showed excellent dose discrimination at ≥ 1 Gy in the time window of 1 to 7 days after exposure except 1 Gy at day 7. Saa1 dose response was limited to the first two days after exposure. A multiplex assay with both proteins showed improved dose classification accuracy. Our magneto-nanosensor assay demonstrates the dose and time responses, low-dose sensitivity, small volume requirements, and rapid speed that have important advantages in radiation triage biodosimetry.
Figures

References
-
- Blakely W. F., Salter C. A. & Prasanna P. G. S. Early-response biological dosimetry— recommended countermeasure enhancements for mass-casualty radiological incidents and terrorism. Health Phys. 89, 494–504 (2004). - PubMed
-
- Pinto M. M. P. L., Santos N. F. G. & Amaral A. Current status of biodosimetry based on standard cytogenetic methods. Radiat. Environ. Biophys. 49, 567–581 (2010). - PubMed
-
- Fenech M. Current status, new frontiers and challenges in radiation biodosimetry using cytogenic, transcriptomic and proteomic technologies. Radiat. Meas. 46, 737–741 (2011).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

