AKI: a path forward
- PMID: 23868899
- PMCID: PMC3805072
- DOI: 10.2215/CJN.06040613
AKI: a path forward
Abstract
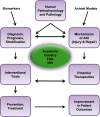
The Kidney Research National Dialogue, supported by the National Institute of Diabetes and Digestive and Kidney Diseases, asked the scientific community to formulate and prioritize research objectives that would improve our understanding of kidney function and disease. High-priority objectives for AKI were identified, including enhancing training and collaborative opportunities, improving phenotyping and development of clinical tools, expanding our understanding of the pathophysiology and interaction between injury and reparative processes, and identifying determinants of long-term outcomes. Research in animal models must be translated to improve diagnosis and treatment of patients. The objectives identified by the Kidney Research National Dialogue provide the research community with future opportunities for improving the prevention, diagnosis, and treatment of people with AKI.
Figures
References
-
- Waikar SS, Liu KD, Chertow GM: Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol 3: 844–861, 2008 - PubMed
-
- Palevsky PM, Molitoris BA, Okusa MD, Levin A, Waikar SS, Wald R, Chertow GM, Murray PT, Parikh CR, Shaw AD, Go AS, Faubel SG, Kellum JA, Chinchilli VM, Liu KD, Cheung AK, Weisbord SD, Chawla LS, Kaufman JS, Devarajan P, Toto RM, Hsu CY, Greene T, Mehta RL, Stokes JB, Thompson AM, Thompson BT, Westenfelder CS, Tumlin JA, Warnock DG, Shah SV, Xie Y, Duggan EG, Kimmel PL, Star RA: Design of clinical trials in acute kidney injury: Report from an NIDDK workshop on trial methodology. Clin J Am Soc Nephrol 7: 844–850, 2012 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

